AF is associated with excess stroke risk due to various mechanisms better explained with Virchow’s triad of deranged blood constituents, vessel wall abnormalities and abnormal blood flow.1 Hence, a vital aspect in the management of patients with AF includes the identification of non-low-risk patients using risk stratification tools, such as the CHA2DS2-VASc score, to identify those who may derive a net benefit from anticoagulation therapy, aimed at preventing thromboembolic complications.2
The benefit of anticoagulation in AF has long been established.3 In fact, a previous meta-analysis of warfarin treatment in patients with AF demonstrated significant reductions of stroke risk and all-cause mortality by 64% and 26%, respectively, compared with placebo or no treatment.4 Yet, a significant proportion of patients with AF remain at high residual stroke risk despite receiving appropriate dose-adjusted anticoagulation therapy, and some patients go on to suffer from anticoagulation-resistant stroke. This represents a clinical conundrum for physicians, particularly as contemporary guidelines in AF are silent in this regard, reflecting the limited evidence surrounding the topic.5–8 This review explores the residual stroke risk in AF and potential therapeutic options for high-risk patients.
Residual Stroke Risk in AF
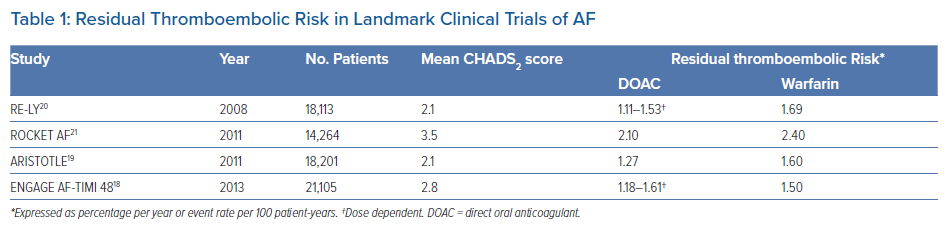
To begin with, it is important to recognise that anticoagulation therapy reduces, but does not negate, the risk of stroke in AF. Recent estimates among patients with AF found that the annual incidence of stroke or systemic embolism with warfarin was 1.66%, an improvement compared with previous reports of 2.09%, which the authors attributed to better quality of anticoagulation control.9 There was a significant increase in the annual incidence of stroke or systemic embolism in patients with additional concomitant risk factors (CHADS2 score ≤1: 0.89% per year; CHADS2 score 2: 1.43% per year; CHADS2 score ≥3: 2.50% per year).9 Notably, the quality of anticoagulation control in that meta-analysis of eight randomised controlled trials, determined using time in therapeutic range (TTR) as between 55% and 68%, remained suboptimal based on the recommendations of >70% according to current international guidelines.7–9
Given the importance of anticoagulation control, efforts were directed at achieving and maintaining a high TTR (>65–70%), especially in high-risk subgroups with AF, in order to improve clinical outcomes.10 However, the introduction of direct oral anticoagulants (DOACs) has changed the landscape of treatment in patients with AF such that TTR is a distant memory in those treated with these newer agents, although compliance continues to be an issue.11,12 Overall, DOACs have been shown to be more effective than warfarin for the prevention of stroke or systemic embolism, even in various high-risk AF subgroups, such as patients with concomitant heart failure, valvular heart disease and coronary artery disease.13–16 In addition, DOAC therapy may reduce stroke severity compared with warfarin.17
Nonetheless, in landmark clinical trials evaluating the different DOAC agents against warfarin among patients with AF, the residual risk of stroke or systemic embolism despite anticoagulation treatment was between 1.11% and 2.40% per year (Table 1).18–21 Similar, if not higher, rates of between 1.73% and 2.78% were reported in real-world studies.22 This residual risk should not be underestimated because the threshold for consideration of oral anticoagulation in AF is approximately 0.9%, corresponding to a CHA2DS2-VASc score of 1, for the risk of thromboembolism. Therefore, there is a need for greater awareness among clinicians and better risk stratification of residual stroke in patients with AF.
There are several potential mechanisms of stroke despite anticoagulation, including small vessel disease, intracranial or extracranial atherosclerotic disease, cryptogenic stroke, arterial dissection and hypercoagulable states (e.g. inherited thrombophilia, antiphospholipid syndrome). Medication errors have also been previously reported to be common among patients with AF.23 Furthermore, there are other causes of cardioembolic stroke besides AF, such as mitral stenosis, mechanical heart valves and left ventricular thrombus. An in-depth review of these factors has previously been published.24 Importantly, anticoagulation therapy has not been proven to be beneficial for stroke prevention in most of these conditions despite an excess risk of bleeding.
Risk Factors for Residual Stroke Risk
AF is a multimorbid condition that is predisposed by the presence of risk factors such as advancing age, hypertension, diabetes, chronic kidney disease, heart failure and coronary artery disease.25,26 In addition, AF accelerates the progression of disease for many of these risk factors.26–28 Hence, AF rarely occurs in isolation, and concomitant diseases may influence the residual stroke risk either by their individual effects, synergism with AF or by reducing the effectiveness of anticoagulation therapy. Numerous risk factors have been shown to be associated with an increased residual stroke risk in AF. However, there are currently no risk stratification tools that have been validated for the prediction of residual stroke risk in anticoagulated patients with AF. As a result, the identification of high-risk patients can be difficult, especially in the busy clinical environment.
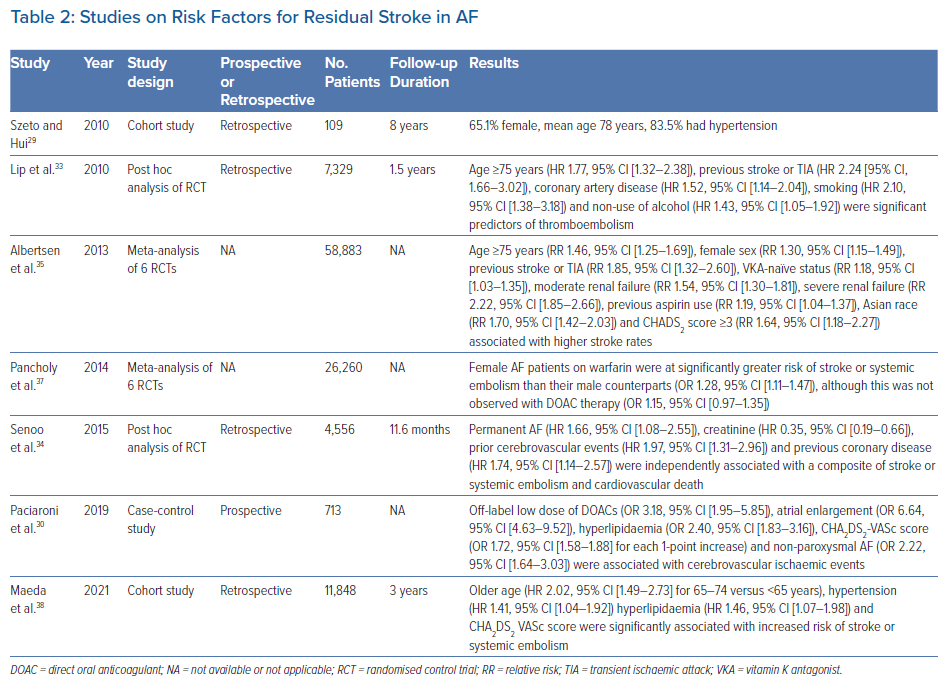
Recently, a cohort study of patients who suffered from stroke despite DOAC therapy identified that most of these patients were older, female and hypertensive, with at least Stage 2 chronic kidney disease (estimated glomerular filtration rate <90 ml/min; Table 2).29 However, that study by Szeto and Hui was limited by its retrospective design and small sample size.29 A prospective case-control study showed that the use of off-label low-dose DOACs, atrial enlargement, hyperlipidaemia, a high CHA2DS2-VASc score and non-paroxysmal AF were independently associated with an increased risk of stroke events among AF patients.30 Main contributors from the CHA2DS2-VASc score were increasing age, diabetes, congestive heart failure and prior stroke or transient ischaemic attack (TIA). Unlike in the study of Szeto and Hui, female sex and hypertension were not found to be independent risk factors.29,30 Interestingly, Paciaroni et al. reported that approximately 30% of patients with cerebrovascular events had stroke due to causes other than cardioembolism; this reinforced the concept that ischaemic stroke in patients with AF is not exclusively cardiogenic in nature.30–32
A post hoc analysis of the SPORTIF trials demonstrated that age ≥75 years, coronary artery disease, smoking and non-use of alcohol were significant predictors of thromboembolism.33 Using data of anticoagulated patients with AF from the AMADEUS clinical trial, Senoo et al. demonstrated worse outcomes of stroke or systemic embolism, and death among those with permanent AF, prior cerebrovascular events, coronary artery disease and impaired renal function.34 However, the results of that post hoc analysis should be interpreted with caution given the historical nature of the trial even though event outcomes were adjudicated.33
A meta-analysis of three randomised controlled trials focusing on warfarinised patients with AF found that age ≥75 years, female sex, prior stroke or TIA, vitamin K antagonist-naïve status, moderate or severe renal failure, previous aspirin use, Asian race and a CHADS2 score ≥3 were associated with higher stroke rates.35 Nonetheless, given the known importance of the quality of anticoagulation control and the fact that it could not be assessed within different subgroups of that meta-analysis, it was unclear whether the predictors identified were directly related to stroke risk or indirectly via their influence on anticoagulation control.36 In addition, the effects of these risk factors on DOAC therapy remained untested within that study.35 In contrast, Pancholy et al. performed their meta-analysis using patients from both treatment groups (warfarin and DOAC) and demonstrated that female patients with AF who were treated with warfarin had a greater residual risk of stroke than their male counterparts, but no sex differences were observed with DOACs.37
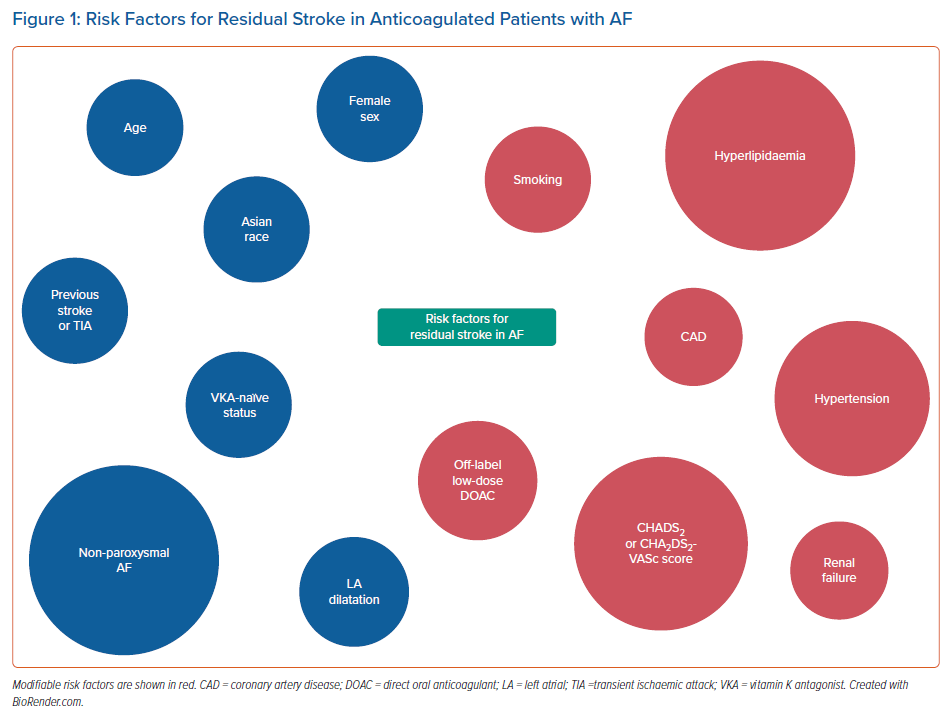
Recently, a retrospective cohort study reported that the risk of ischaemic stroke or systemic embolism among patients with AF remained markedly higher than that of the general population even after anticoagulation therapy, as observed previously.38,39 Furthermore, approximately one-third of the residual risk was secondary to modifiable factors, including hypertension, diabetes and hyperlipidaemia.38 Overall, there appears to be an overlap between many of the risk factors for residual stroke risk in AF (Figure 1) and those that predispose to stroke events in non-anticoagulated AF patients.40 Nonetheless, the former remains poorly defined and further studies are needed to determine the extent and mechanisms by which these risk factors affect residual stroke risk in AF.
Potential Treatment Options
Despite some awareness of residual stroke risk in AF, this issue presents a clinical problem to physicians because there is little evidence on effective management strategies for patients recognised to be at high risk. In this regard, several studies have explored the use of antiplatelet agents in addition to anticoagulation to further minimise stroke risk in AF. However, this approach should not be advocated for the management of residual stroke risk among the general AF population given the increased risk of harm and lack of demonstrable benefit.41,42
There is growing evidence to suggest that AF patients who have previously suffered a stroke despite anticoagulation are at increased risk of subsequent strokes compared to anticoagulation-naïve patients, highlighting the importance of managing the residual risk of ischaemic stroke in AF.43–45 There are several other strategies that have shown positive results for this purpose, including catheter AF ablation, left atrial appendage (LAA) occlusion and adherence to theAF Better Care (ABC) pathway. It is important to highlight that none of these approaches has been specifically proven for the management of residual stroke risk in AF.
Catheter AF Ablation
Contemporary international guidelines recommend rhythm and/or rate control strategies for symptom management in patients with AF.6,8 This was on the basis of historical studies performed over a decade ago that failed to demonstrate any prognostic advantage of one over the other.46–48 Nonetheless, the results of these studies were confounded by the fact that there were high rates of anticoagulation discontinuation during follow-up among patients who were randomised to receive a rhythm control strategy. A post hoc analysis of the ATHENA trial suggests that a rhythm control approach in addition to usual care may reduce stroke events among patients with AF.49 Recent evidence demonstrates that early rhythm control, by any means, in AF was beneficial in reducing cardiovascular events (including stroke) compared with rate control.50
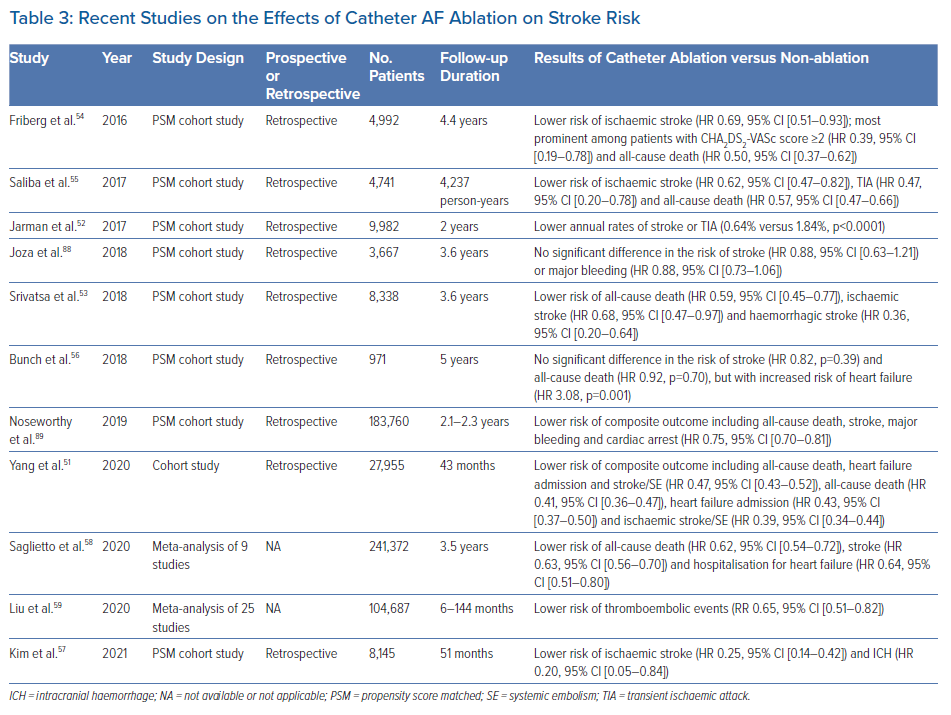
AF ablation is a means of rhythm control and an established treatment for patients with drug-refractory symptomatic AF. Although not used for the sole purpose of risk modification, several observational cohort studies have reported that catheter AF ablation was independently associated with a lower risk of ischaemic stroke, the effects of which were more pronounced among patients with higher CHA2DS2-VASc score (Table 3).51–55 In a study of patients with AF and prior stroke, Bunch et al. found that patients who underwent catheter AF ablation had lower rates of recurrent stroke over a 5-year period compared with those who were not ablated.56 Notably, the long-term rates of recurrent stroke were comparable between ablated AF patients and non-AF patients.56 Similar results were obtained in a nationwide study using the Korean National Health Insurance Service (NHIS) database.57
Recent meta-analyses reinforced that catheter AF ablation significantly reduces the risk of thromboembolism compared with medical therapy alone.58,59 Importantly, the vast majority of patients in these studies were anticoagulated, suggesting that catheter AF ablation may have a role as adjunctive therapy in the management of residual stroke risk in AF. A further advantage of this strategy is that it may be combined with LAA occlusion (discussed below) in a single procedure.60 Overall, although catheter AF ablation has shown promise, it is not currently indicated for the reduction of stroke risk in AF because it remains unclear whether this approach may interrupt the natural history of AF and/or cause a significant alteration in the subsequent stroke risk, despite the results from observational studies. This is supported by the lack of a temporal relationship between AF episodes and stroke complications, and an increased risk of thromboembolic events with traditional risk factors, even in the absence of AF.1,61,62 Moreover, the recent CABANA trial failed to demonstrate a significant benefit of catheter ablation over drug therapy for the composite endpoint of all-cause death, disabling stroke, serious bleeding or cardiac arrest in the intention-to-treat analysis.63
For patients who undergo catheter AF ablation, there is some debate as to whether anticoagulation therapy is necessary among those with successful maintenance of sinus rhythm after the initial prothrombotic phase post-ablation. In the landmark AFFIRM trial, patients randomised to the rhythm control arm exhibited a trend towards a greater risk of ischaemic stroke that largely occurred following discontinuation of anticoagulation therapy, indicating that the decision for anticoagulation should be guided by stroke risk factors rather than the perceived success of maintaining sinus rhythm.47 This observation may be due, in part, to undetected, asymptomatic recurrences that commonly occur in the postablation period, often found only with more aggressive monitoring strategies.64
Lately though, there is some evidence to suggest that the stroke risk in AF is significantly lowered by catheter ablation such that the risk-to-benefit ratio may favour the suspension of oral anticoagulation following a successful procedure.65 A meta-analysis of seven retrospective cohort studies demonstrated that the withdrawal of anticoagulation 3 months after successful radiofrequency catheter AF ablation was associated with a significant reduction in the risk of haemorrhage, and no difference in thromboembolic events at both short- and long-term follow-up.66 Nonetheless, this is a topic that warrants further investigation. Presently, the decision of whether to anticoagulate patients with successful catheter AF ablation should continue to be guided by individual stroke risk factors, as per contemporary international guidelines.7,8
Left Atrial Appendage Occlusion
The LAA is an important structure in AF because the majority of cardioembolic stroke originates from here.67–69 Therefore, occlusion of this structure acts to isolate and prevent clot formation and subsequent embolisation. LAA occlusion may be performed using either a surgical or percutaneous approach. The latter is emerging as a viable treatment alternative to anticoagulation in AF, although more research is required to define its exact role.70
A meta-analysis of five randomised controlled trials of patients with AF or risk factors for AF comparing LAA occlusion to standard of care using anticoagulation therapy (in the era of warfarin) found that LAA occlusion was at least non-inferior for stroke prevention with a potential for reduction in mortality.71 The PRAGUE-17 randomised control trial demonstrated that among AF patients with a high risk of stroke (mean CHA2DS2-VASc score 4.7), LAA occlusion was non-inferior to DOAC therapy in preventing major AF-related cardiovascular, neurological and bleeding events.72 These results were reaffirmed in a real-world registry of AF patients with a very high stroke risk (CHA2DS2-VASc score ≥5) and ‘unacceptable’ risk of bleeding, where the residual annual ischaemic stroke risk was 2.8% after LAA occlusion.73 Furthermore, data from the Amplatzer Cardiac Plug registry suggest that LAA occlusion may be an effective treatment option for secondary stroke prevention in AF patients with anticoagulation-resistant stroke.74 Nonetheless, the retrospective nature of that small observational study should be acknowledged.
Importantly, none of the aforementioned studies showed that treatment with LAA occlusion was superior to anticoagulation for stroke prevention in AF. Recently, the LAAOS III trial reported that among patients with AF who had undergone cardiac surgery, the risk of ischaemic stroke or systemic embolism over a follow-up period of 3.8 years was lower with concomitant LAA occlusion than without it.75 The vast majority of patients who received LAA occlusion remained on anticoagulation therapy, proving the notion that there is a combined effect of LAA occlusion and anticoagulation for stroke prevention in AF. Overall, the use of LAA occlusion as add-on therapy to anticoagulation in AF for patients with high residual stroke risk remains to be proven, although it may offer some hope in desperate situations.76,77
AF Better Care Pathway
The ABC pathway was introduced as a means to facilitate an integrated management of patients with AF in a holistic manner.78 It was founded on three main principles: ‘A’, avoid stroke; ‘B’, better symptom management; and ‘C’, cardiovascular and comorbidity optimisation. Post hoc analysis of the AFFIRM trial showed that an integrated care approach based on the ABC pathway was associated with a significant decrease in the composite risk of stroke, major bleeding and cardiovascular death compared with non-ABC care.79 However, this finding was largely driven by a reduction in the risk of major bleeding and cardiovascular death, because stroke risk was not statistically different between the groups. Similarly, the risk of stroke was unchanged in AF patients with clinical management adherent to the ABC pathway from the ESC-EORP Atrial Fibrillation General Long-Term Registry.80
In contrast, nationwide cohort studies of AF patients from the Korean NHIS database demonstrated a significant reduction in the rates of ischaemic stroke with implementation of the ABC pathway.81,82 In addition, the mAFA-II trial found that patients who were randomised to mobile health management based on the ABC pathway (versus usual care) had lower rates of ischaemic stroke.83 Overall, differences in the results of the aforementioned studies may be related to the methods in which the ABC pathway was evaluated and patients were deemed to be ABC adherent. A recent meta-analysis of the ABC pathway in AF showed that this strategy was associated with a 45% reduction in the risk of ischaemic stroke, indicating the benefit of this approach in the management of residual stroke risk in AF.84
Management of Residual Stroke Risk
It is recommended that the management of patients with AF includes a holistic approach by combining patient education, lifestyle modification, psychosocial management and strategies that promote medication adherence.8 Hence, I propose that the management of residual stroke risk in AF should incorporate the implementation of an integrated, multidisciplinary care strategy with clear communication between healthcare professionals and a structured approach (Table 4). In this regard, the importance of appropriate administration of dose-adjusted anticoagulation therapy in AF should not be overlooked because the use of off-label doses has been linked to poorer outcomes.85,86 The detection and management of modifiable risk factors associated with AF, such as hypertension, coronary artery disease, heart failure, diabetes, hyperthyroidism, obesity and valvular heart disease, must also be prioritised. An in-depth review of this has been published elsewhere.87 Moreover, other sources of stroke risk should be considered, because some may have a major effect on overall management strategies.24 Given the limited evidence in this area, the decision to pursue specific treatment options such as catheter AF ablation and LAA occlusion to minimise residual stroke risk in AF should be individualised.
Conclusion
Residual stroke risk among anticoagulated patients with AF represents a real challenge in the clinical environment. Presently, the identification and subsequent management of high-risk individuals are poorly explored topics. Future studies are needed to define risk factors of residual stroke in AF and determine the effects of specific treatments in this patient cohort.
Clinical Perspective
- A significant proportion of patients with AF remain at high residual stroke risk despite receiving appropriate dose-adjusted anticoagulation therapy.
- Risk factors for residual stroke risk in AF may include age, female sex, Asian race, previous stroke or TIA, VKA-naïve status, non-paroxysmal AF, left atrial dilatation, smoking, hypertension, hyperlipidaemia, coronary artery disease, renal failure, CHADS2 or CHA2DS2-VASc score, and use of off-label low-dose DOAC.
- The management of residual stroke risk should incorporate an integrated multidisciplinary care strategy, with clear communication between healthcare professionals and a structured approach.