Transcatheter aortic valve implantation (TAVI) has become the standard therapy in patients with symptomatic severe aortic stenosis and an intermediate or high surgical risk.1,2 Recent promising reports suggest that it will also become standard therapy in low-risk patients.3,4 New conduction disturbances following the procedure requiring permanent pacemaker implantation (PPI) have been well described.5,6 Although mortality and major complication rates have decreased with the newer-generation valves, the rate of pacemaker implantation and conduction disturbances remains high with both self-expandable (SE) and balloon-expandable (BE) valves.7–11 Furthermore, there are reports of the development of delayed conduction abnormalities and late complete atrioventricular block (CAVB).12–15 Recent studies have shown that PPI after TAVI was associated with a lower risk of sudden cardiac death (SCD) at 1 year follow-up.16,17 The use of implantable loop recorders (ILR) revealed that 20% of patients with new-onset persistent left bundle branch block (LBBB) after TAVI had severe bradyarrhythmias, with half of them requiring PPI during the 1-year follow-up.18 An early discharge approach (≤72 hours) after TAVI is increasingly being used, including continuous ECG monitoring <48 hours that may lead to underdiagnosis of conduction and rhythm abnormalities.19–24 Tools to identify the subgroup of patients at higher risk of developing late conduction disturbances are needed. Whereas guidelines for PPI are relatively straightforward for patients with documented second degree or higher atrioventricular (AV) conduction disorders, there is no consensus for PPI in patients who develop new-onset LBBB with or without PR prolongation after TAVI.25,26 The lack of guideline recommendations in patients with relative indications such as LBBB with or without PR prolongation has led to a centre-based approach, which varies significantly among different centres and ranges from PPI to a watchful waiting strategy, with some centres performing further evaluation with electrophysiological studies (EPS) or ILRs.24
The value of the His–ventricular (HV) interval in assessing the risk of developing high-degree AV block (AVB) in patients with chronic degenerative conduction disease and bundle branch block was described by Scheinman et al. in 1982; an HV interval ≥70 ms was found to carry a fourfold increased risk of developing CAVB, whereas HV ≥100 ms identified a subgroup at particularly high risk (25%).27 Katritsis and Josephson found that approximately 70% of patients with HV intervals ≥100 ms develop second- or third-degree infra-His block within the next 2 years.28
Currently, there are on-going clinical trials evaluating the utility of EPS in patients undergoing TAVI.29
This review summarises contemporary data on the use of EPS as a predictor of PPI and as a tool for decision making regarding the need for PPI in patients undergoing TAVI.
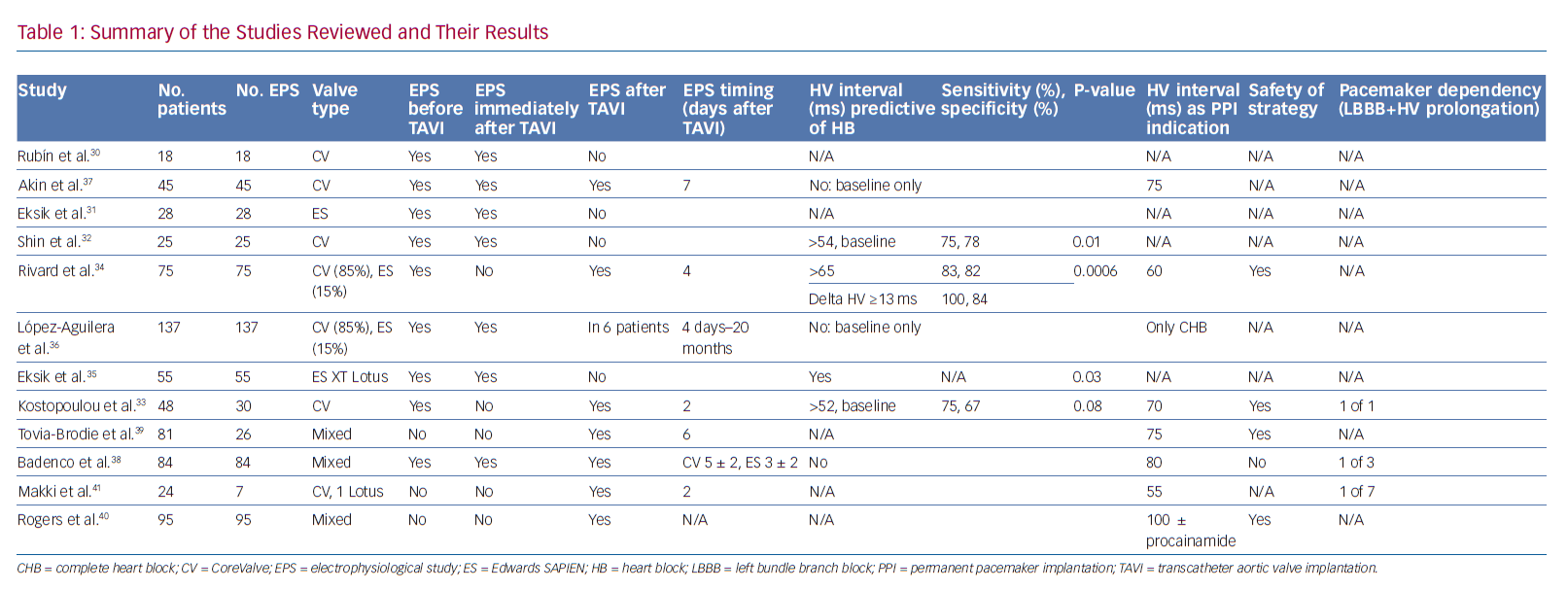
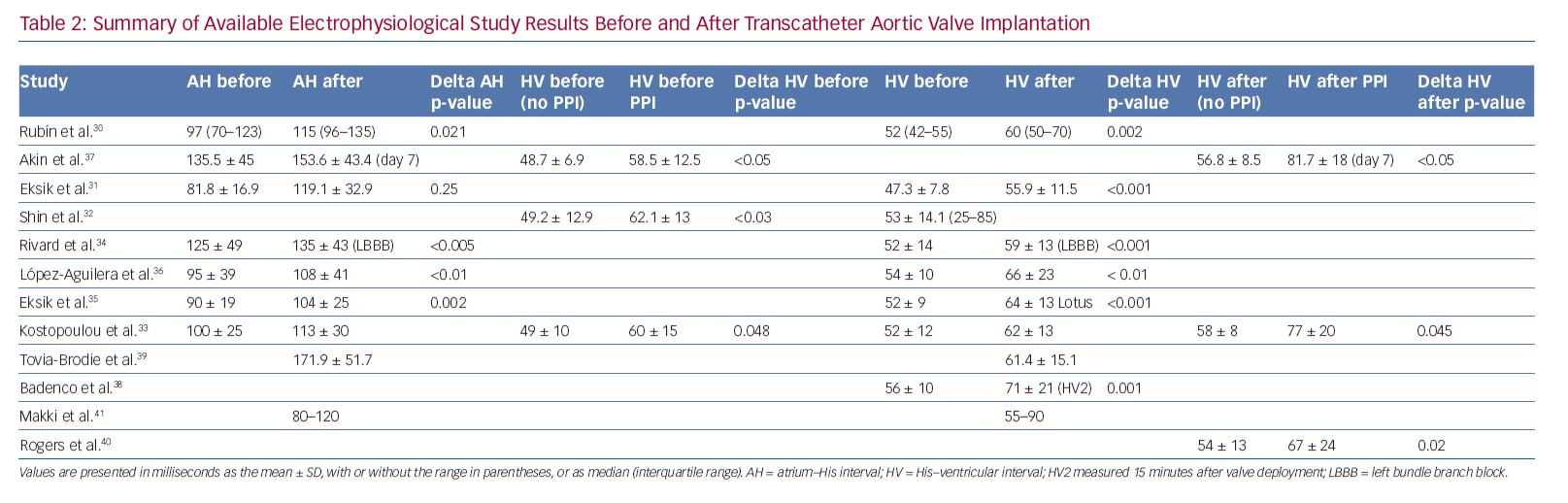
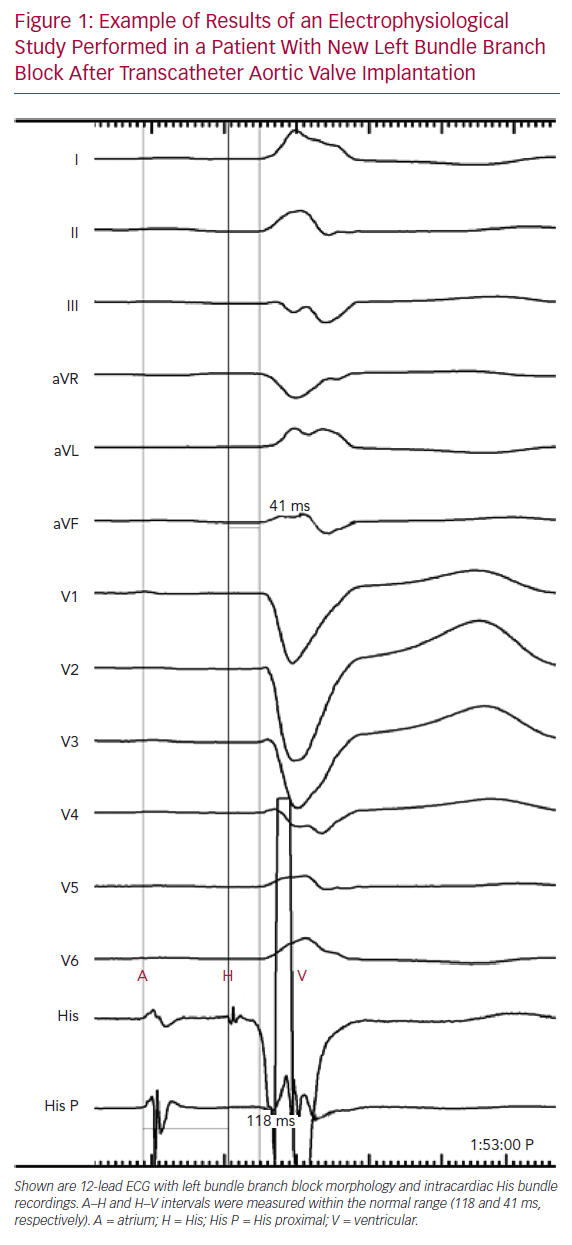
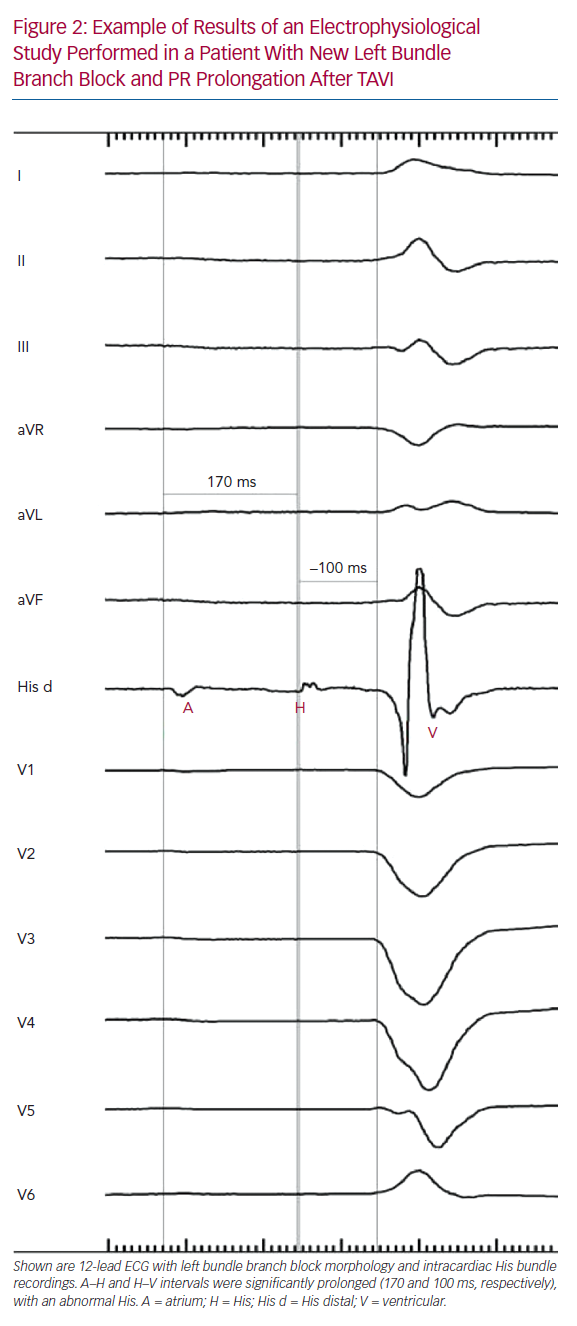
Tables 1 and 2 summarise the studies and their results; Figures 1 and 2 are examples of EPS results demonstrating normal and abnormal atrium–His (AH) and HV intervals in patients with post-TAVI new conduction disturbances.
Predictive Value of Electrophysiological Studies
Before and After TAVI Without Analysis of Predictors of Permanent Pacemaker Implantation
Several studies have assessed EPS results before and after TAVI. Rubín et al. reported the results of 18 patients who underwent EPS immediately before and immediately after CoreValve (Medtronic, Minneapolis, MN, US) prosthesis implantation: the HV interval was significantly prolonged after TAVI.30 On follow-up, one patient who developed a new LBBB after TAVI and had a post-TAVI prolonged HV interval (76 ms) experienced recurrent syncope after discharge; paroxysmal CAVB was documented in this patient 10 days after TAVI, and a permanent pacemaker was implanted. All four patients who underwent PPI after TAVI had a normal pre-TAVI EPS. However, the study had no statistical power to identify predictors of AVB because of its small sample size.30
Eksik et al. reported the results of 28 patients who underwent EPS immediately before the initial balloon valvuloplasty and immediately after implantation of an Edwards SAPIEN prosthesis (Edwards Lifesciences). 31 In these patients, the HV interval increased significantly after the procedure. Conduction disturbances were observed in EPS and ECG immediately after the procedure in 10.7% of patients, but these disturbances recovered before discharge.31 Moreover, the conduction defects were mainly infranodal and temporary. Only one patient with right bundle branch block (RBBB) and left anterior hemiblock required PPI (3.6%); in this patient, the HV interval increased from 45 to 75 ms. Predictors of AV conduction problems could not be analysed because of the small sample size and low PPI rate.31
Electrophysiological Studies Before TAVI
Studies Predicting Permanent Pacemaker Implantation
Shin et al. reported the results of 25 patients who received a CoreValve prosthesis.32 EPS was performed before and after TAVI if no CAVB occurred. Patients developing CAVB had a significantly longer HV interval at baseline than patients with no indication for PPI. Furthermore, an HV interval >54 ms at baseline showed a predictive value for the development of CAVB after TAVI with a sensitivity of 75% and a specificity of 82.4% (95% CI [0.542–0.902]), reaching statistical significance (p=0.009).
Kostopoulou et al. reported the results of 48 patients who underwent a CoreValve implantation and were randomised to ECG plus EPS evaluation or to ECG evaluation only.33 Thirty patients in the EPS group underwent a baseline EPS followed by TAVI and a second EPS 48 hours after the procedure. The indication for PPI was the combination of new LBBB with infrahisian conduction delay, which was defined as an HV interval >70 ms. Five of the 30 patients in the EPS group developed CAVB immediately after TAVI and therefore did not undergo repeat EPS. Patients with baseline conduction abnormalities (HV interval >50 ms) were at higher risk of developing post-TAVI CAVB. In the two patients who developed CAVB relatively late (after day 2) and underwent a post-TAVI EPS, the HV interval increased significantly (to >70 ms). Receiver operating characteristic (ROC) analyses indicated an HV interval of 52 ms (sensitivity 75%, specificity 67%) as a cut-off value that showed a trend for PPI (HR 4.054, 95% CI [0.816–20.138]; p=0.087). Of the 14 patients with complete new LBBB, only one needed PPI. This patient developed first-degree AVB and LBBB on day 2, which progressed to CAVB on day 7. All patients with new LBBB were in the non-EPS group. Univariate but not multivariate analysis identified prolonged baseline HV interval as a significant factor associated with PPI after TAVI, whereas delta HV was not significantly associated with PPI. There were no patients with a normal post-TAVI EPS who underwent PPI over the long-term follow-up.33
Studies Not Predicting Permanent Pacemaker Implantation
López-Aguilera et al. reported the results of 137 patients who underwent CoreValve prosthesis implantation and were studied by EPS before and 30 min after valve implantation.36 Mean AH and HV intervals increased significantly (p<0.01). Furthermore, baseline intracardiac intervals did not predict the need for post-TAVI PPI within 72 hours. Post-TAVI EPS results were not included in the analysis.36 Six patients required an additional repeat EPS after valve implantation, which was performed between day 4 and 20 months after TAVI. Two patients experienced considerable deterioration in AV conduction after valve implantation that returned to normal on repeat EPS 5 and 7 days after TAVI. Three patients experienced symptoms during the long-term follow-up. Repeat EPS showed high-degree AVB with considerable AH interval prolongation at 16 months of follow-up in one patient, and sick sinus syndrome in the remaining two patients 1 and 20 months after TAVI. The remaining six patients had sinus rhythm with first-degree AVB and significant prolongation of AH and HV intervals. During the first 24 hours, paroxysmal CAVB was observed. Repeat EPS 4 days after TAVI showed complete infrahisian AVB and a permanent pacemaker was implanted.36
Akin et al. reported the results of 45 patients who underwent CoreValve implantation.37 EPS was performed prior to valve implantation, immediately after valve implantation and at the 7-day follow-up. PPI was indicated in the presence of new LBBB in combination with HV interval prolongation ≥75 ms. Patients who underwent PPI (n=23; 51%) had significantly longer PQ, AH and HV intervals in all EPS. Eighteen of 22 patients with first-degree AVB had a prolonged HV interval (13 of 22 [59%] with HV prolongation ≥75 ms). The HV interval increased from baseline to immediately after TAVI, and further at 7 days after TAVI (mean ± SD 58.5 ± 12.5, 74.0 ± 13.5 and 81.7 ± 17.8 ms, respectively) and was significantly higher in patients receiving PPI than in those not.37 A multivariate analysis to identify predictors for high-grade AVB revealed new LBBB immediately (within 60 minutes) after TAVI (OR 24.85, 95% CI [1.57–392.57], p=0.023), PQ interval >200 ms (OR 11.37, 95% CI [1.138–97.620[, p=0.02) and QRS interval >120 ms (OR 14.28. 95% CI [1.50–135.88]. p=0.021) as predictors for high-grade AVB. Other clinical parameters and baseline ECG parameters (e.g. AH interval >100 ms, HV interval >75 ms, QRS interval >120 ms and PQ interval >200 ms) had no ability to predict critical conduction delay.37 Post-TAVI EPS data were not included in this analysis.
Summary and Interpretation
The predictive value of pre-TAVI EPS has shown mixed results. Although two studies did not find a correlation between baseline HV interval and PPI,36,37 another two studies found baseline HV intervals of 52 and 54 ms to be predictive of higher risk for PPI.32,33 These values are still within the normal HV interval range, and the implementation of these cut-off values as a strategy for PPI would result in a high rate of PPI, the majority of which are not needed.
Predictive Value of Electrophysiological Studies Before and After TAVI
Studies Predicting Permanent Pacemaker Implantation
Predictive Value of Delta His–Ventricular Interval
Rivard et al. reported the results of 75 patients who were implanted with CoreValve (85%) or Edwards SAPIEN (15%) valves and underwent EPS at baseline and after TAVI (at a median of 4 days after the procedure).34 In patients with new-onset LBBB, there was a significant increase in both AH and HV intervals after compared with before TAVI. In multivariate analysis, the delta HV interval was the only factor independently associated with CAVB (HR 1.152 per ms, 95% CI [1.063–1.248]; p=0.0006). ROC analysis revealed that the sensitivity and specificity for predicting CAVB were 100% and 84.4%, respectively, for a delta HV interval of ≥13 ms and 83.3% and 81.6%, respectively, for an HV interval of ≥65 ms after TAVI. Negative and positive predictive values were 100% and 70%, respectively, for a delta HV interval of ≥13 ms and 82% and 62%, respectively, for an HV interval of ≥65 ms after TAVI. Excluding the EPS results before TAVI from multivariate analysis, the only factor independently associated with CAVB was delta QRS duration (HR 1.060 per ms, 95% CI [1.024–1.097]; p=0.001). A delta QRS duration of ≥38 ms was associated with a sensitivity and specificity of 88.9% and 76.3%, respectively. In patients with new-onset LBBB, the HV interval after TAVI and the delta HV interval, but not delta QRS duration or delta PR interval, were associated with CAVB in univariate analysis. The delta HV interval was the only factor independently associated with AVB (HR 1.152 per ms, 95% CI [1.063–1.248]; p=0.007) in multivariate analysis.34
Predictive Value of Electrophysiological Studies After TAVI
Eksik et al. reported the results of 55 patients who were randomised to implantation of an Edwards SAPIEN XT or Lotus (Boston Scientific) prostheses.35 EPS before and immediately after valve implantation was performed. The AH and HV intervals increased significantly after Lotus implantation. In the case of Edwards SAPIEN XT implantation, post-TAVI HV intervals were similar to those before the procedure. However, both the post-TAVI QRS duration (mean ± SD 138 ± 26 versus 116 ± 28 ms; p=0.004) and HV interval (mean ± SD 64 ± 13 versus 55 ± 11 ms; p=0.02) were significantly longer in the Lotus than SAPIEN XT group. In multivariate analysis, a significant association was noted between pre-TAVI AV conduction disorders and post-TAVI HV interval and the adjusted risk of PPI (post-TAVI HV OR 1.156, 95% CI [1.012–1.319]; p=0.033).
Rivard et al.34 also performed multivariate analysis without including the pre-TAVI EPS results and found that the HV interval after TAVI was the only predictor of CAVB (HR 1.081 per ms, 95% CI [1.014–1.152]; p=0.0163) with a sensitivity and specificity of 80.0% and 77.8%, respectively, for a cut-off value of ≥65 ms.
Electrophysiological Studies Not Predicting Permanent Pacemaker Implantation
Badenco et al. reported the results of 84 patients implanted with CoreValve (66.7%) or Edwards SAPIEN (33.3%) prostheses. EPS was performed immediately before TAVI (defined as ‘HV1’) and 15 minutes after valve deployment (‘HV2’). A third EPS was performed after TAVI (3 ± 2 days for Edwards SAPIEN, and 5 ± 2 days for CoreValve) in 64 patients (‘HV3’).38 A PPI was implanted if HV3 was >80 ms regardless of ECG conduction disturbances. The mean HV2 interval was significantly prolonged compared with HV1. In all, 19 patients experienced high-degree AVB before discharge: nine patients with persistent CAVB during TAVI and 10 patients with new AVB from day 2 to day 6 (eight CoreValve, two Edwards SAPIEN). All these patients had perioperative conduction disturbances, including transient CAVB (n=4) or new LBBB (n=6). Neither HV1, HV2 interval nor delta HV2–HV1 were correlated with CAVB after TAVI. Pre-existing RBBB and perioperative persistent CAVB were the main factors predicting early postoperative CAVB in univariate analysis (p=0.001 and p<0.001, respectively) and only perioperative persistent CAVB remained statistically significant in multivariate analysis (p=0.001). The mean HV3 interval decreased to 63 ± 14 ms (p=0.002). HV3 >70 ms and the delta HV3–HV1 (mean 13 ± 5.5 ms) were not correlated with delayed AVB (p=0.84 and p=0.4, respectively). In 14 patients studied on days 4–7, HV measured 70–100 ms, but no AVB developed. Nine of these 14 patients were implanted for HV3 >80 ms, of whom four had no conduction disturbances, two had transient periprocedural AVB and only three had new LBBB. Conversely, three patients developed high-degree AVB (on days 2, 4 and 7) when their HV3 interval remained unchanged from HV1 and HV2, below 80 ms.
Summary and Interpretation
The available studies are relatively small, with differences in valve type and EPS timing. All studies demonstrated a significant increase in HV interval after TAVI. When a third EPS was performed during hospitalisation (the second EPS being immediately after TAVI), the results varied from a further increase to a partial decrease in HV interval.37,38 These differences may be explained by different valve types, the timing of EPS and earlier versus later experience with TAVI. The predictive value of pre- and post-TAVI EPS and delta HV has shown more consistent results in predicting the need for PPI, except for one study. That study found that delta HV was the only factor independently associated with CAVB (HR 1.152 per ms).34 In addition, delta HV ≥13 ms (sensitivity and specificity 100% and 84%, respectively) and HV interval ≥65 ms (sensitivity and specificity 83% and 82%, respectively) predicted PPI. When excluding the pre-TAVI EPS results from the analysis, a post-TAVI HV interval of ≥65 ms (HR 1.081 per ms; sensitivity and specificity 80.0% and 77.8%, respectively) predicted PPI.34 Another study found post-TAVI HV interval to be predictive of PPI with an OR of 1.156.35 Conversely, another study found no correlation between HV interval or delta HV and PPI using three EPS.38
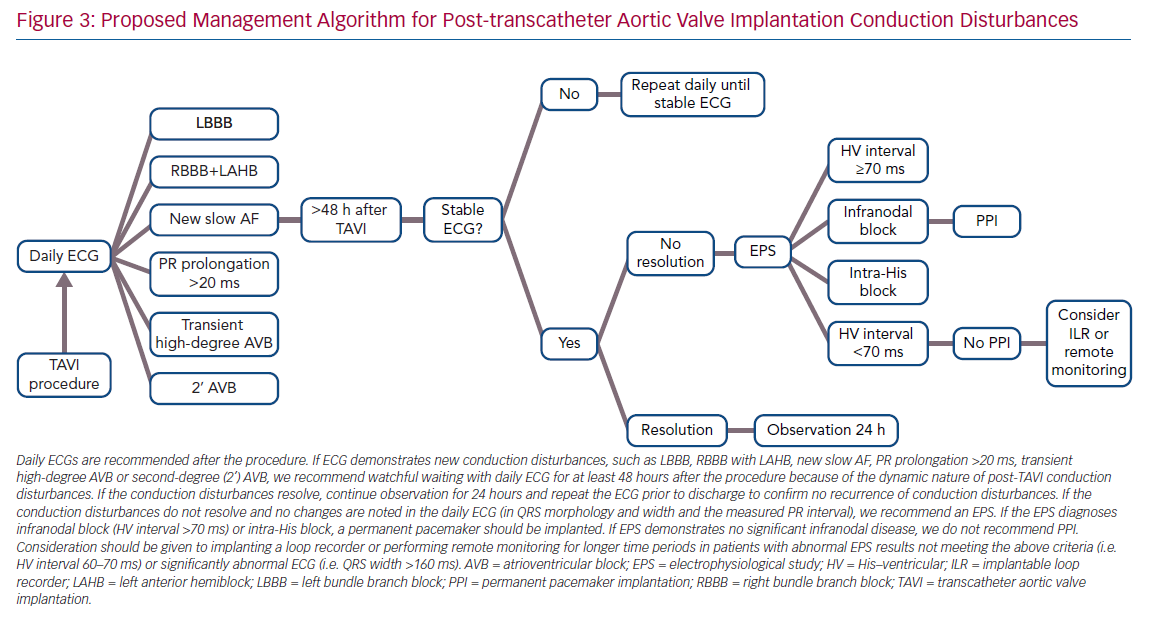
The differences in results may be explained by the different timing of the EPS. The mechanism of AVB development after TAVI is thought to be the direct compression of the prosthesis on the His bundle and AV node.42 Hence, the design and expansion properties of the valve have a direct effect on the risk and timing of the development of new conduction disturbances. The self-expanding property of the CoreValve’s prosthesis led to the maintenance of a steady radial force on the annular and subendocardial tissue, presumably for several days after implantation.43,44 The mechanically expanded Lotus valve has a braided Nitinol frame and a polyurethane/polycarbonate outer seal, and has demonstrated similar effects on the conduction system.35,45,46 The new-generation Edwards SAPIEN 3 valve has an adaptive external polyethylene terephthalate fabric seal designed to minimise paravalvular leaks.9 All these designs result in continuation of the radial pressure caused by the prosthesis over a time period exceeding the TAVI procedure. Combining these valve mechanisms with the occurrence of late conduction disturbances after TAVI and the finding that conduction disturbances continue to progress during the first few days after the procedure, and begin to recover after days 4–6,47,48 have led to two main recommendations:39
- All patients should be evaluated with a daily ECG.
- The EPS should be performed only after the conduction disturbances in the ECG have stabilised, preferably prior to discharge.
Post-TAVI Electrophysiological Study-guided Permanent Pacemaker Implantation Strategy
Tovia-Brodie et al. presented the results of 81 patients with conduction abnormalities after TAVI with either BE or SE valves.39 A total of 26 of these patients underwent post-TAVI EPS. Indications for PPI were severe infranodal conduction disturbances, such as greater than first-degree intra-His block, HV interval >75 ms and the occurrence of second-degree infranodal block during incremental atrial pacing at a cycle length >400 ms. Multilevel conduction disturbances involving the AV node (n=19; 73.1%) and the His (n=3; 11.5%) and infra-His system (n=4; 15.4%) were found. Eight patients (30.8%) in the EPS group received PPI. There were five (9%) deaths and three (5.5%) PPIs after discharge among patients who did not undergo EPS, and none in the EPS group. The cause of death was SCD in three patients, heart failure in one patient and an unknown reason in another patient. The reason for PPI was CAVB in two patients and syncope associated with new LBBB with PR prolongation in one patient. The pacemakers were implanted 29, 96 and 252 days (mean 126 ± 114.4 days) after the procedure. Accordingly, undergoing an EPS was independently associated with prolonged event-free survival (defined as PPI or death; 100% versus 85.4%; p=0.04), but not overall survival (100% versus 90.9%; p=0.12).
Rogers et al. reported EPS results of 95 patients who underwent TAVI with either BE or SE valves and developed conduction disturbances without an absolute indication for PPI.40 If a subject had intra- or infra-hisian block, or the HV interval was >100 ms at baseline, the EPS was considered positive. Otherwise, patients were challenged with IV procainamide (dose ranging from 500 mg to 1 g) administered over 10 minutes. If the HV interval increased to >100 ms or if the patient developed intrahisian or greater than second-degree infrahisian block with procainamide challenge, the EPS was considered positive. The final decision to implant a permanent pacemaker was left to the discretion of the electrophysiologist. The only significant difference between patients who had a positive EPS and underwent a PPI to those with a negative EPS was the mean post-TAVI HV interval (67 ± 24 versus 54 ± 13 ms; p=0.02). There was no significant difference in 30-day and 1-year mortality between patients who received a permanent pacemaker and those who did not.40 After an initial cautious implementation, the number of EPS procedures performed increased significantly. This increase corresponded with a decline in the overall PPI rate. Hospital length of stay in patients with a negative EPS and no PPI was equivalent to that in patients with no conduction disturbance. None of the patients with a negative EPS required PPI after hospital discharge during the 1-year follow-up after TAVI, whereas 2% of patients who had no conduction abnormalities after TAVI (and therefore did not undergo an EPS) needed PPI at 1-year follow-up. Rogers et al. concluded that an EPS-guided PPI strategy is safe for the management of conduction disturbance after TAVI.40 In patients with equivocal indication for pacing after TAVI, EPS avoided PPI in over 70% of patients.
Summary and Interpretation
The available studies are relatively small and different HV intervals were used as an indication for PPI. Only one study reported the occurrence of high-degree AVB in three patients after the decision was made not to implant a pacemaker based on post-TAVI EPS during follow-up.38 That study used a relatively high cut-off of HV interval >80 ms as an indication for PPI, without the use of a drug challenge for stressing the conduction system. The three cases occurred on days 2, 4 and 7 after the procedure, and no details were provided as to valve type, the timing of the EPS (possibly too early if CoreValve was used and the EPS was done prior to the development of the block) or whether the HV interval measured was normal (HV <55 ms) or prolonged (HV 55–79 ms), making the interpretation of these results difficult. In two studies, the only two patients needing PPI due to a relative indication demonstrated significant infranodal conduction disturbances in combination with HV interval ≥75 ms.30,31 In the remaining four studies that reported the results of long-term follow-up and the need for PPI in patients, with the decision made not to implant based on post-TAVI EPS results, no SCD occurred and none of the patients needed a PPI during at least 1 year of follow-up.33,34,39,40 Even though event-free survival (including late PPI or SCD) was 100% in the EPS-guided PPI group and few events occurred in the patient group discharged without an EPS, the difference in mortality and PPI rate did not reach statistical significance. This can be explained by the relatively small number of patients and low event rates. Based on these studies, when using EPS-guided PPI strategy, the cut-off value for HV interval should be in the range 65–75 ms (when a drug challenge is not used) or 100 ms with a drug challenge.
As emphasised previously, the authors do not recommend the use of ajmaline or flecainide as substitutes for procainamide in the drug challenge during EPS in post-TAVI patients.39 These patients frequently have severe left ventricular hypertrophy or dysfunction and may be at high risk of developing complications, especially life-threatening proarrhythmic events, following administration of ajmaline
or flecainide.49
Pacemaker Dependency
Makki et al. reported the results of 24 patients who underwent in-hospital PPI after TAVI (mainly with CoreValve prostheses) and had a follow-up of at least 3 months.41 PPI was indicated in the presence of LBBB with an abnormal HV interval >55 ms or elicitation of CAVB at EPS. Seven patients were implanted due to LBBB and an abnormal HV interval on EPS. The HV interval ranged from 55 to 90ms. The single patient with the borderline HV (55 ms) who developed CAVB during EPS was the only patient of the seven to remain pacemaker dependent at follow-up. Pacemaker dependency was defined as >50% pacing upon device interrogation, underlying complete heart block, underlying asystole >5 seconds or symptoms in the setting of bradycardia (rate <50 BPM).
Badenco et al. reported on pacemaker dependency.38 AVB was assessed in the pacemaker memory with a specific AVB management algorithm and the rate of ventricular pacing (a rate of 2% was considered relevant for analysis). During follow-up, high-degree AVB persisted in 12 of 17 patients implanted for perioperative AVB and in one of nine patients implanted prophylactically due to an increased HV3 interval. Six of these nine patients were implanted without persisting new conduction disturbances after the procedure based on HV interval measurements only.
Kostopoulou et al. reported the follow-up results of pacemaker interrogation at 1 month after TAVI.33 Nine patients were implanted due to CAVB and one was implanted due to LBBB with an HV interval of 70 ms. The devices were programmed in an endogenous preference mode to be able to evaluate pacemaker dependency and the percentage of pacing on the next assessment of the device. Pacemaker dependency was defined as asystole or complete heart block with or without escape rhythm after cessation of pacing. Four of 10 patients (25% of first-year implantations) remained pacemaker dependent, including the one patient implanted because of LBBB with HV prolongation. Two of three patients with documented infrahisian conduction delay with an HV interval >70 ms underwent PPI and remained pacemaker dependent throughout follow-up.
Summary and Interpretation
Not surprisingly, pacemaker dependency in patients implanted for a relative indication (LBBB plus prolonged HV interval) is lower than in patients implanted due to CAVB, involving three of 11 patients (27%), combining data from all studies (Table 1). However, the interpretation of device interrogation data regarding PPI necessity is problematic. Pacemaker dependency definition varied between studies. Many studies rely on the percentage of ventricular pacing as a surrogate for heart block and pacemaker dependency. However, devices are not always programmed meticulously to prefer intrinsic rhythm conduction, nor can a ventricular pacing rate <1% exclude short episodes of CAVB, because most devices implanted do not use an algorithm to identify the underlying rhythm. In addition, most device interrogations are performed 1-month after TAVI; hence, the exact timing of the recovery of intrinsic rhythm is not known. Considering all of this, it is the authors’ opinion that PPI in this subgroup of patients with new conduction disturbances and a prolonged HV interval cannot be avoided in order to try to prevent SCD in these patients, while acknowledging not all SCD are necessarily the result of bradyarrhythmias that could have been avoided by pacing. The authors’ proposed management algorithm for post-TAVI conduction disturbances, without an absolute indication for PPI, is shown in Figure 3.
Conclusion
As the indications for TAVI are expanding, more patients will experience post-procedural conduction disturbances and the dilemma of PPI will be encountered more frequently. EPS may be a useful tool for evaluating these patients. There is a general agreement that the HV interval increases after TAVI, especially in patients with new conduction disturbances. The use of a post-TAVI EPS-guided PPI strategy in patients with new conduction disturbances is the one presently adopted by the authors. HV interval cut-off values for PPI ranging from 65 to 75 ms (or 100 ms with procainamide drug challenge) appear to be safe. The timing of EPS should depend on the stabilisation of the conduction disturbances on the ECG. Further large-scale studies including the various newer-generation valve types are needed to further validate this approach.
Footnote
A comprehensive review on the management of conduction disturbances associated with TAVI has recently been published by Rodés-Cabau et al.50 In that review, the use of EPS is suggested as an option for the management of patients with new LBBB or aggravation of pre-TAVI conduction disturbances. Infra-His block or an HV interval >100 ms (without drug challenge) are suggested as indications for PPI. Importantly, however, this cut-off value was not used in any of the studies using EPS-guided PPI strategy in post-TAVI patients.
Clinical Perspective
- Conduction disturbances remain a frequent complication after transcatheter aortic valve implantation (TAVI), especially with newer-generation valve prostheses.
- Prolongation of the His–ventricular interval after TAVI was predictive of permanent pacemaker implantation in several studies.
- Based on the results of relatively small studies, electrophysiological studies may be useful for evaluating patients who develop post-TAVI conduction disturbances.