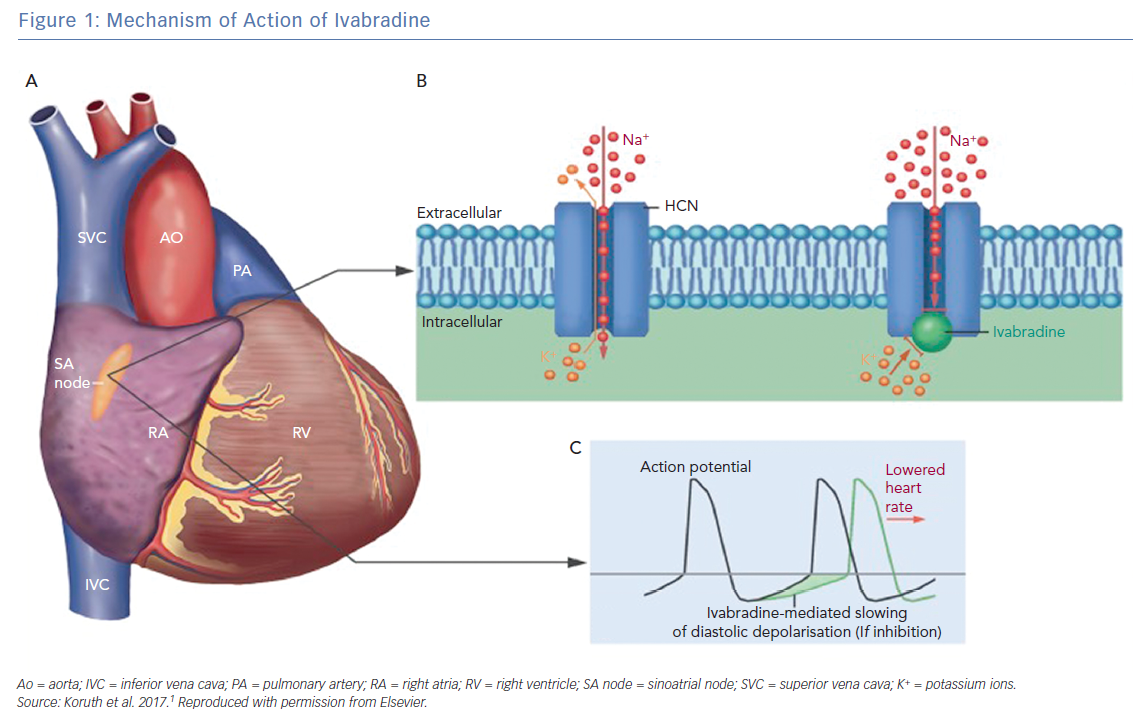
Ivabradine is a pure heart rate-lowering agent best characterised by its negative chronotropic effect on the sinoatrial node.1 Its unique mechanism selectively blocks the pacemaker funny (If) channels, which are responsible for spontaneous depolarisation in the sinoatrial node that regulates heart rate during sinus rhythm (Figure 1).2 Since 1980, it has been well established that controlling the heart rate is the main target when treating coronary artery disease (CAD) and heart failure (HF) and is associated with a beneficial effect on mortality and morbidity.3,4
Current Approved Clinical Indications
According to the European Society of Cardiology guidelines for heart failure, ivabradine should be considered in order to reduce the risk of hospitalisation due to HF or cardiovascular death in symptomatic patients with left ventricular ejection fraction (LVEF) ≤35%, a sinus rhythm and resting heart rate of ≥70 BPM despite treatment with beta-blockers, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker and mineralocorticoid receptor antagonist.5 Ivabradine should be considered for the same indication in patients who are not able to tolerate beta-blockers or have contraindications. In these patients, angiotensin-converting enzyme inhibitor (or angiotensin receptor blocker) and mineralocorticoid receptor antagonist should also be given. While for the treatment of stable angina with symptomatic HF with reduced ejection fraction, ivabradine should be considered as an antianginal agent in patients with sinus rhythm and heart rate of ≥70 BPM as per recommended management in combination with beta-blockers or when beta-blockers are not tolerated.
According to the 2017 update to the American College of Cardiology/American Heart Association and the Heart Failure Society of America guidelines for the management of HF, ivabradine can be useful to reduce hospitalisation for HF in patients with symptomatic stable chronic HF with reduced ejection fraction (LVEF ≤35%) who are receiving guideline-based treatment, including beta-blockers at a maximum tolerated dose, and who are having sinus rhythm with heart rate of ≥70 BPM at rest.6
Ivabradine in Induction of AF
The If current, which is affected by ivabradine, was found to be present in the pulmonary vein myocardial sleeves, the well-recognised triggers for AF.7 This may explain the risk of AF in patients receiving this drug. However, AF is commonly associated with HF and ischaemic heart disease, the current two clinical indications for the use of ivabradine, hence AF in this patient population may be an association rather than a drug-induced effect.8,9 The increased incidence of AF in patients receiving ivabradine for heart rate control in the setting of acute coronary syndromes or HF was a major concern in several previous trials.
Early clinical studies of ivabradine, such as the International Trial on the Treatment of Angina With Ivabradine versus Atenolol (INITIATIVE) study and the Morbidity-mortality Evaluation of the If Inhibitor Ivabradine in Patients with Coronary Disease and Left Ventricular Dysfunction (BEAUTIFUL) trial focused on its effect on heart rate to control chest pain as an antianginal agent.10,11 The later Systolic Heart Failure Treatment with the If inhibitor ivabradine (SHIFT) trial, which included HF patients, showed a greater reduction in adverse events in patients with HF who received ivabradine. Considering the mechanism of action of ivabradine, the expected side-effect would be sinus bradycardia and not AF.12
However, the Study Assessing the Morbidity-Mortality Benefits of the If Inhibitor Ivabradine in Patients With Coronary Artery Disease (SIGNIFY), which is the largest randomised controlled trial involving coronary artery disease patients without HF, showed that frequency of AF and bradycardia were significantly higher in the ivabradine arm when compared with placebo.13 The SIGNIFY subgroup analyses, which were published later, showed that neither AF nor bradycardia were related to adverse events.14 A meta-analysis of 11 studies investigating the risk of AF with ivabradine treatment has shown that ivabradine treatment is associated with a 15% increase in the relative risk (RR) of AF. Furthermore, 208 patient years of treatment with ivabradine is required to cause one new case of AF. Some of the data on AF in this meta-analysis were obtained via personal communication.15
Another meta-analysis of the risk of AF with ivabradine treatment, which included eight randomised controlled trials (n=36,501), showed that the incidence of AF was 5.34% (n=1,126) in the ivabradine group and 4.56% (n=885) in the placebo group. There was a significantly higher incidence of AF (24% RRI) in the ivabradine group when compared with placebo (RR 1.24; 95% CI [1.08–1.42] p=0.003).16
Ivabradine in Maintenance of Sinus Rhythm
Data from several studies showed that ivabradine may have a role in the maintenance of sinus rhythm. A small study (65 patients) published in 2015 demonstrated that ivabradine added to amiodarone was more efficient than amiodarone alone in the maintenance of sinus rhythm in patients with left ventricular diastolic dysfunction and persistent AF who underwent sinus rhythm restoration, with similar rates of adverse events in both groups.17 The efficacy and safety of ivabradine in the maintenance of sinus rhythm in patients undergoing cardiac surgery were also addressed in a study by Iliuta et al. involving 527 patients undergoing elective valve replacement, coronary artery bypass grafting (CABG) or both.18 Patients had to have left ventricular systolic dysfunction, conduction abnormalities (defined as first degree atrioventricular [AV] block, left bundle branch block, bifascicular or trifascicular block) or both to be included in the study. Perioperatively, patients received metoprolol 100 mg per day, ivabradine 5 mg twice daily, or a combination of metoprolol 50 mg once daily and ivabradine 5 mg twice daily, and were followed for 30 days after the operation. The results revealed a lower incidence of postoperative AF in the combination therapy group (8.94%) than either metroprolol alone (9.66%) or ivabradine alone (19.77%; p<0.001). The combination therapy group also had a lower incidence of third-degree AV block and worsening heart failure (6.15% and 4.47%, respectively) than the metoprolol-only group (12.5% and 7.95%, respectively), while the incidence of such events was lowest in the ivabradine-only group (2.91% and 2.33, respectively).18
In 2016, a study by Abdel-salam et al. showed similar results. They randomised 740 patients undergoing elective CABG with or without valve replacement to perioperative administration of bisoprolol alone, ivabradine alone or a combination of both. Patients with an LVEF of <45% or prior history of AF or atrial flutter were excluded. The results demonstrated a reduced incidence of postoperative AF in the combination therapy group (4.2%) compared with the ivabradine-only group (15.1%) and the bisoprolol-only group (12.2%). The results were statistically significant (p<0.001).19
The electrophysiological basis of using ivabradine for sinus rhythm maintenance may be explained by the changes that occur in the distribution of the channels that maintain the If current, termed the hyperpolarisation-activated cyclic nucleotide-gated (HCN) cation channels. Lai et al. found a significantly higher level of If gene expression in the free walls and appendages of both atria in patients with AF undergoing CABG compared with those without AF.20 HCN messenger RNA was also found to be significantly more abundant in the right atrial appendage samples of aged patients (34 patients) with AF compared with aged patients in sinus rhythm.21 On the other hand, another study showed a reduced level of HCN messenger RNA in the right atrial appendage of patients with chronic AF undergoing heart surgery compared with those in sinus rhythm. However, HCN protein expression showed no significant difference between the two groups, while the If current was higher in the AF group.22 These findings suggest that the If current plays a role in the complex pathophysiological procedure that initiates and maintains AF. If current inhibition by ivabradine may thus have a role in the prevention of AF.
Ivabradine in Rate Control
The heart rate-lowering effect of ivabradine was recently demonstrated in animal models with AF. Meanwhile, Moubarak et al. reported adequate control of heart rate (by 24-hour ECG monitoring) in a patient with permanent AF and an ejection fraction of 35% who was receiving ivabradine for heart failure and no concomitant rate-lowering drugs.23 This effect was confirmed by repeating the 24-hour ECG monitoring 1 week after stopping ivabradine therapy, showing a significant rise in the mean heart rate (80.1 BPM on ivabradine versus 87.6 BPM without ivabradine). Another case report showed a decrease in both the mean heart rate (84 BPM versus 102 BPM) and maximum heart rate during exercise (153 BPM versus 169 BPM) in a patient with an LVEF of 35% and permanent AF upon the initiation of ivabradine therapy.24 These were shortly followed by an open-labell trial that involved adding ivabradine therapy to six symptomatic patients with persistent or permanent AF who were already receiving maximum-tolerated doses of beta-blockers and had a resting heart rate of >110 BPM. This study demonstrated a significant reduction in both the mean resting heart rate (86.3 BPM at 3 months versus 109.5 BPM at baseline) and the maximal heart rate (143 BPM at 3 months versus 178 BPM at baseline) after the initiation of ivabradine therapy.25 Such findings clearly justified the conduction of a randomised controlled double-blind trial. In 2017, Wongcharoen et al. randomised 31 patients with non-paroxysmal AF who were already on standard rate-lowering therapy (beta-blockers, calcium channel blockers and digoxin) to ivabradine add-on therapy (n=21) versus placebo (n=11). There was a significant reduction in the 24-hour mean heart rate in the ivabradine group (86.0 ± 10.9 BPM at baseline to 79.2 ± 9.6 BPM after ivabradine, p<0.001). Ivabradine also showed a statistically significant reduction in the mean heart rate compared with placebo (6.9 ± 6.3 BPM with ivabradine versus 1.4 ± 6.0 BPM with placebo, p=0.024).26
The newly discovered use of ivabradine as rate-control therapy was extended to involve another subgroup of patients. In 2017, Fontenla et al. published a case study in which a patient with a prosthetic mitral valve, permanent AF, left bundle branch block and severe LV systolic dysfunction had previously received a cardiac resynchronisation therapy defibrillation (CRT-D) device follow-up showed a 74% of biventricular pacing due to rapid conduction of AF over the AV node. The patient was already on a maximum tolerated dose of beta-blockers and had a history of digitalis toxicity. The administration of 5 mg of ivabradine twice daily raised the percentage of biventricular pacing to 95%, providing a good alternative to AV-nodal ablation.27 Based upon these findings, a randomised controlled multicentre clinical trial: the IvaBRAdine blocK of Funny Current for Heart Rate Control in permanEnt Atrial Fibrillation (BRAKE-AF Study; NCT03718273), is currently being conducted to evaluate the rate-lowering effect of ivabradine on patients with permanent AF. In this study, patients with symptomatic permanent AF who are receiving maximum-tolerated doses of beta-blockers or calcium channel blockers and showing evidence of poorly controlled heart rate, are being randomised to therapy with either ivabradine or digoxin. The primary endpoints of this study are heart rate reduction and adverse events. The results of the confrontation between the 23-year-old drug ivabradine and the 200-year-old drug digoxin would certainly be interesting and would hopefully shed light on the efficacy and safety of ivabradine therapy in the large and overgrowing cohort of patients with AF. Given the evidence that we have, off-label use of ivabradine as an add-on therapy for rate control might be reasonable in selected patients after explaining the risks and benefits.
The mechanism by which ivabradine achieves heart rate control is not completely understood. However, since HCN channels are not exclusive to the sinoatrial node and are also expressed in the AV node and the conduction system, albeit at a lower density, the effect of ivabradine on the HCN channels in the AV node is possibly the mechanism by which it lowers the heart rate during AF.28
Ivabradine for Other Indications
Postural orthostatic tachycardia syndrome (POTS) is a form of orthostatic intolerance that usually occurs in younger adults and children. Patients frequently report palpitations, presyncope, and fatigue. The hallmark of this disorder is an exaggerated heart rate increase in response to postural change without arterial hypotension. Although optimal therapy of POTS is not established. It is currently recommended to avoid precipitating factors, encourage physical activity and volume repletion. Other options include fludrocortisone, beta-blockers and midodrine.29 Unfortunately, even after implementing all conventional therapy, fewer than 60% of patients report improvement of their symptoms.30 Given that the primary problem is an accelerated heart rate, several authors investigated ivabradine’s role in POTS. In a retrospective case series that included 22 patients, 60% of patients treated with ivabradine reported symptomatic improvement.31 In another prospective study that included eight patients diagnosed with POTS, ivabradine slowed the heart rate of POTS patients at rest by 4 ± 1 BPM and during a 5-minute head-up tilt, heart rate decreased from 118 ± 4 BPM to 101 ± 5 BPM (p<0.01).32 Given that there is limited data on ivabradine’s efficacy in POTS, a randomised controlled trial to access its efficacy is needed to further evaluate its role in this syndrome.
Ivabradine has also been found to be effective in controlling the heart rate, termination of tachycardia as well as maintenance of sinus rhythm in patients with incessant atrial tachycardia.33–35 Recently, a small study conducted on 28 patients with incessant focal atrial tachycardia showed that ivabradine successfully achieved complete termination of the tachycardia (17 patients) or adequate reduction in the heart rate without a change in rhythm (one patient). Tachycardias originating from the atrial appendages were more likely to respond to ivabradine.36
Conclusion
Ivabradine is a unique medication which has an approved indication for heart rate reduction in heart failure with reduced ejection fraction and angina in selected patients. Previously, ivabradine’s heart rate reduction was thought to be exclusively due to inhibition of If channels in the sinoatrial node. However, emerging data has shown channels that maintain the If current in the free wall of both atria. These findings support the idea that the If current plays a role in the complex pathophysiological procedure that initiates and maintains AF. So far, there is evidence that ivabradine triggers AF and can contribute to the maintenance of sinus rhythm in people with AF. In addition, since those channels are expressed in the AV node and the conduction system as well, some studies have shown that ivabradine may have a role in rate control of AF as an add-on therapy. Given the diversity of the data, further randomised prospective studies are needed before recommending an expanded role for ivabradine.