Late gadolinium enhancement (LGE) cardiac MRI is increasingly used to detect cardiac fibrosis in the context of arrhythmias.1–4 Fibrosis is a hallmark of arrhythmogenic cardiac remodelling and constitutes an important substrate in both atrial and ventricular arrhythmias.2,3
Of note, by exploiting the slow washout kinetics of gadolinium in extracellular space, LGE-MRI is not only capable of determining native fibrotic tissue, but also of detecting ablation-induced scarring.5–13 Several groups have reported LGE-MRI-based localisation of functional gaps in atrial ablation lesions with high accuracy, and even LGE-MRI-guided repeat pulmonary vein isolation (PV) has been demonstrated to be efficient and effective as a standalone approach.5,7,14 Although the use of LGE-MRI for ventricular ablation lesion assessment is lagging behind compared to the atrium, feasibility has been demonstrated in preclinical and early clinical studies.10,15,16 However, initial data on the accuracy of LGE-MRI-based lesion assessment were somewhat conflicting.5,6,17,18 This limited reproducibility of promising results across centres may have been because of differences in the methods of image acquisition, post-processing and analysis.19 In addition, we have generated evidence that the timing of image acquisition with respect to different stages of lesion formation and scar remodelling also has to be considered.20
This review focuses on the assessment of chronic ablation lesions using LGE-MRI and its utility in clinical practice. The scope of this article does not include real-time monitoring of lesion formation through intraprocedural MRI, which – despite holding great promise – is still of limited clinical relevance because of current structural and technical limitations related to electromagnetic interference, as well as the relative absence of acute parameters that accurately predict definite lesion formation.21
Pathology of Lesion Formation
The pathology of radiofrequency (RF) ablation injury is well established in animal models and patients and is characterised by coagulation necrosis, haemorrhage and complete loss of cellular and vascular architecture, apart from a narrow peripheral transition zone.9,22–25 The response to ablation injury implies infiltration of immune cells and neovascularisation, with local inflammation and interstitial oedema being observed up to 8 weeks post-ablation.11,23,25 In parallel, activated fibroblasts proliferate and differentiate into myofibroblasts that generate fibrogenic signals, which perpetuate tissue repair and promote collagen deposition resulting in replacement of myocardium with fibrous scar tissue.22,24–26 While RF ablation and cryoablation fundamentally differ in the acute effect on the tissue and the mechanism of cell death, most of these basic principles of RF-ablation-induced scar formation appear to apply similarly to cryoablation, albeit less well established and with an arguably more preserved ultrastructural tissue integrity.27,28
It has been shown that scar formation and remodelling in response to MI is a dynamic and chronically sustained process that continues over years after the initial injury.29,30 Once recruited to injured myocardium, fibroblasts persist in the infarct scar for years where they continue to generate fibrogenic signals that perpetuate tissue repair and promote fibrosis.29,30 While data from long-term longitudinal studies on ablation-induced scarring are lacking, a recent analysis of post-mortem cardiac samples from patients with previous ventricular tachycardia (VT) ablation, indicates that we have to consider such long-term remodelling processes also in response to catheter ablation.25
LGE-MRI for the Detection of Ablation-induced Fibrosis
Basic Principles
LGE-MRI for the detection of myocardial fibrosis was first used and histologically validated in a canine model of MI.31 Despite pathophysiological differences, both cardiac ablation and MI result in coagulation necrosis, loss of syncytial membrane integrity and eventually replacement fibrosis. LGE-MRI makes use of the expansion of extracellular space and thus increased volume of distribution for the contrast agent that is associated with replacement fibrosis, as well as the prolonged washout owing to decreased capillary density within the myocardial fibrotic tissue.32,33 Gadolinium-based contrast agents diffuse freely into the interstitium, but they cannot cross intact cell membranes and thus accumulate in the extracellular space. As gadolinium contrast agents reduce the T1 relaxation time of adjacent tissue, LGE enhancement results in an increased signal intensity in T1-weighted MRI sequences. It is noteworthy though that LGE is not specific for fibrotic tissue, but can reflect other pathological processes associated with an expansion of the extracellular space such as inflammation and oedema formation, which impedes definite lesion assessment, particularly in the acute setting.34
Timing of Gadolinium Application
As indicated above, besides interstitial volume of distribution, LGE is determined by wash-in and washout kinetics of the contrast agent. Thus, the exact time delay between contrast administration and image acquisition is critical. While image acquisition is typically performed 7–15 minutes (ventricle) or 15–25 minutes (atrium) post contrast agent injection for differential spatial contrast between scar and normal tissue, there is no consensus among different centres. Moreover, in some cases the time delay may even be adapted, based on individual perfusion (cardiovascular function) and washout kinetics (renal function). At our centre, we acquire atrial images 20 minutes after an intravenous bolus of 0.2 mmol/kg of gadobutrol, whereas in the ventricle we acquire images 7–10 minutes after gadolinium injection.
Of note, the time to allow for gadolinium to enter lesions appears to be of particular relevance regarding the so-called dark core phenomenon. This phenomenon is characterised by a hypoenhanced region (dark core) within ablation lesions surrounded by a peripheral rim of LGE.11,15,16,35 The exact pathological correlate underlying this phenomenon remains unknown, but microvascular obstruction impeding gadolinium wash-in is likely to play a role (no reflow). Possibly owing to a lack of functional capillaries, gadolinium appears to enter ablation lesions via diffusion from the lesion periphery.9,11 This hypothesis is based on the centripetal expansion of LGE towards the lesion centre resulting in a diminishment of the hypoenhanced dark core, which is observed with increasing time delays between gadolinium administration and image acquisition allowing for longer diffusion times.9,11
Contrast Agent and Dosage
The impact of different gadolinium-based contrast agents and doses on image quality are of particular interest in light of the recent safety concerns, not only regarding the induction of nephrogenic systemic fibrosis but also with respect to cerebral gadolinium deposits, the long-term clinical relevance of which is still unknown. Against this background, there may be a rationale to reduce doses of gadolinium-based contrast agents. While there has been no head-to-head-comparison of the different agents in the context of cardiac LGE imaging, recent data showed that Gadovist (Bayer) at 0.10 mmol/kg provides inferior LGE image quality than 0.15 and 0.20 mmol/kg with respect to ventricular scar assessment (image acquisition at a median of 9 minutes post gadolinium injection).36 Interestingly, there is evidence suggesting that scans with 3T provide a signal-to-noise ratio that allows for better delineation of scarred myocardium than 1.5 T scans, even with lower contrast concentration (0.10 versus 0.20 mmol/kg Dotarem [Guerbet]), but again, systematic comparative data are lacking.37
While the above-mentioned results obtained for Gadovist and Dotarem, respectively, may not be generalisable to other gadolinium-based contrast agents, it has to be noted that, of the contrast agents presented in this review, only Gadovist and Dotarem are authorised for cardiac MRI in the EU (Table 1).
Image Acquisition
While several protocols have been detailed and validated previously, to date no consensus has been reached regarding standardised image acquisition and magnetic resonance (MR) sequence.9,38–40 Typically 1.5 or 3 T scanners are used for post-contrast image acquisition employing fast 3D gradient echo sequences with ECG-gating and fat suppression. Low flip angles are applied to reduce saturation effects with short repetition times. To further optimise T1 contrast and signal intensities, inversion recovery sequences nullifying the signal of healthy ventricular myocardium are employed. Here, the optimum inversion time (TI) suppressing healthy myocardium (typically 250–300 ms) is determined empirically using a TI scout module prior to the acquisition of definite images. Healthy myocardium will thus appear hypoenhanced relative to scar tissue. TI values may have to be adapted during the scan to accommodate incremental T1 values of the normal myocardium owed to gadolinium washout.
To minimise cardiac motion artefacts, ECG gating is usually performed with the image acquisition window limited to <20% of the RR interval (typically 150–200 ms) and a trigger delay corresponding to atrial or ventricular mid-diastole, sparing atrial and ventricular contraction, respectively. In tachyarrhythmic patients, the trigger delay can be adapted according to the mean RR interval. However, we and others found signal-to-noise ratios substantially reduced in patients with AF.39,41 Therefore, we strongly recommend cardioverting patients prior to the LGE-MRI study to avoid insufficient image quality. Long breath-holds required for image acquisition may be a limiting factor in some patients, a problem that is addressed by free-breathing 3D navigators that suppress respiratory motion artefacts through respiratory gating. Typical LGE-MRI sequences result in a voxel size of 1.25 x 1.25 x 2.5 mm with scan times of 10–15 minutes, depending on heart rate and breathing patterns.
The use of cardiac MRI in patients with implanted cardiac devices has been limited not only because of safety concerns, but also due to hyperintense image artefacts. While numerous studies and the advent of MR conditional cardiac pacemakers and ICDs have largely dispelled the safety concerns, image artefacts have remained a major limitation.42–44 The artefacts are the result of significant distortion of the MRI magnetic field induced by the metallic pacemaker or ICD components.45,46 They are typically located in the proximity (5–10 cm) of the device, with the distance being inversely associated with artefact size.45,46 Of note, the artefacts are particularly pronounced in LGE-MRI sequences as applied for ablation lesion assessment. The use of lower magnetic field strength and shorter echo times has been shown to reduce artefacts, but this may be at the cost of image signal intensity and contrast.46 Recently, based on the hypothesis that the device-related artefacts are caused by the limited spectral bandwidth of the inversion pulse that is typically applied in LGE-MRI, specific wideband MR sequences have been established.47,48 We and other centres are now successfully employing these sequences, enabling high image quality without hyperintensity artefacts, even in the proximity of implanted devices.49
Image Post-processing
Several established open-source and commercial platforms for image post-processing are available. Most of these enable semiautomatic segmentation where manual tracings of the endocardial and/or epicardial borders are automatically adjusted to build a 3D anatomical shell. Relative signal intensities are then colour-coded and projected onto the 3D anatomical shell to create a relative LGE map discriminating healthy myocardium from scar tissue based on predefined thresholds. Some post-processing software even allows for integration of LGE maps into common electroanatomical mapping (EAM) systems.
As mentioned above, to date there is no standardised method for LGE image acquisition, and the same applies to image post-processing and analysis, which may explain the limited reproducibility across different centres. Most importantly, as T1-weighted imaging is based on signal intensity contrast rather than directly measured absolute values, LGE quantification requires a consistent internal reference for normalisation as well as validated signal intensity thresholds discriminating healthy and scar tissue.
While methods using normalisation based on the somewhat arbitrary definition of healthy atrial myocardium have been described for atrial lesion assessment, we and others use the mean signal intensity of the blood pool as an internal reference. Our group has recently established a method quantifying signal intensity ratios using the mean signal intensity of the left atrial (LA) blood pool as a reference (signal intensity of each given voxel/mean signal intensity of the blood).38 Thresholds to define healthy myocardium (signal intensity ratio ≤1.2) and ablation-induced scarring (signal intensity ratio >1.32) in the atrium were derived from distinct cohorts of young healthy individuals as well as post-AF ablation patients, respectively, and subsequently validated in numerous clinical studies with respect to electroanatomical voltage mapping as well as clinical endpoints.5–7 However, it should be emphasised that various other methods using distinct internal references and thresholds have been validated (Table 1).17,39,50,51
For ventricular lesion assessment the ‘full width half maximum’ method is the most commonly used approach, although other methods defining remote ‘healthy’ myocardial segments as an internal reference for normalisation have been described.52–55 We found that the best agreement with electroanatomical voltage mapping was achieved when applying thresholds of >60% (dense scar) and <40% (healthy tissue) of the maximum signal intensity, but again, other thresholds have been proposed too (Table 1).56–59
Timing of Lesion Assessment
As outlined above, we have to assume that ablation lesion formation is a dynamic process of sustained remodelling, which implies constant changes affecting cellular composition, extracellular space, water content and vascularisation, as well as small-vessel permeability and patency and intravascular pressure. These changes in turn, inevitably alter tissue-inherent magnetic properties and wash-in/washout kinetics of gadolium.60,61 In fact, we found determination of definite atrial ablation lesions by LGE-MRI to be more accurate at 3 months post ablation than at later time points >12 months after ablation.20 Thus, it is evident that lesion assessment by LGE-MRI is dependent on the exact time point. Moreover, LGE is not specific for fibrosis and does not necessarily indicate formation of durable ablation lesions. In fact, particularly in the acute setting, LGE may represent oedema reflecting a transient inflammatory response, which usually resolves within the first month following ablation.11,62 In addition, the sensitivity of LGE to detect acute ablation lesions may be locally reduced by the above-mentioned dark core or no reflow phenomena, where limited diffusion times result in central hypoenhanced regions within ablation lesions. As diffusion distances are minimal in the thin-walled atrium, this phenomenon seems to be less relevant in the context of atrial ablation, where central hypoenhanced regions have only been observed in acute lesions.35 Even in the ventricle, these hypoenhanced regions are less frequently encountered in chronic lesions >1 month post-ablation, possibly because of on-going remodelling of scar tissue including neovascularisation, although data are somewhat conflicting in this regard (see the section Ventricular Ablation Lesions, below).11,15,16,63
Taken together, these time-dependent limitations may argue for a late timing of LGE-MRI-based lesion assessment. However, we have recently found a decreased detectability of atrial ablation lesions at very late time-points, >12 months post-ablation, compared with an assessment at 3 months post ablation.20 The long-term decrease in LGE of pulmonary vein (PV)-encircling ablation lesions observed in this study could in theory also reflect true regression of ablation-induced fibrosis; in fact, such a phenomenon has been proposed previously as a possible explanation for non-durable ablation lesions and late AF recurrences. However, using invasive high-density mapping as a reference, we found the decrease in LGE over time to be because of reduced detectability of ablation-induced fibrosis by LGE-MRI at time-points >12 months post ablation. Again, the pathological correlate underlying this observation is unclear, but likely to involve on-going remodelling altering tissue-inherent magnetic properties and wash-in/washout kinetics of the gadolinium contrast agent.
Against this background, the time-point of 3 months post ablation that has become an established standard for lesion assessment with LGE-MRI in many labs, appears reasonable. This time-point has been shown to reliably indicate chronic lesion formation in the atrium and in the ventricle and has been rigorously validated with respect to functional gaps detected by EAM as well as clinical endpoints like AF recurrence.6,62,64,65
Atrial Ablation Lesions
LGE-MRI of the Atrium
While LGE-MRI is a well-established tool to aid or guide VT ablation, its usage for the assessment of fibrosis in the atrium is somewhat lagging behind. This is because of – in part – two reasons. Firstly, unlike the well-demarcated extensive post-MI scar, atrial fibrosis is typically less extensive and more diffuse. This renders detection difficult, as conventional T1-weighted MRI relies on differential spatial contrast between normal tissue on one side and abnormal tissue on the other side. Secondly, differentiation of spatial contrast is particularly difficult in the thin-walled atrium with wall thicknesses down to 1 mm, which approximates the limit of spatial resolution of MRI. However, recent advances in MR imaging techniques, such as 3D navigated inversion recovery sequences, have yielded improved resolution and signal-to-noise ratios enabling valid tissue characterisation also in the atrium.39,66 With respect to atrial ablation lesions it has to be considered that these constitute dense scar, which facilitates discrimination from healthy tissue by LGE-MRI. Thus, even before validation of LGE-MRI for the detection of native atrial fibrosis, it was successfully employed for the assessment of atrial ablation-induced scarring (Figure 1).40,64,67
While initial studies evaluating the capability of LGE-MRI to accurately localise functional gaps within ablation lesions yielded conflicting results, depending on the performing centre, it has to be taken into account that time-points and protocols for image acquisition as well as post-processing methods varied substantially in these studies.5,6,17,18 As outlined above, this may account for the lack of reproducibility. Promoted by further technological and methodological advances in the last decade, LGE-MRI is now being established as a useful standard for risk stratification, patient selection and lesion assessment in the context of AF ablation in a growing number of specialised centres.2
Of note, the feasibility of lesion assessment with LGE-MRI has been demonstrated, both in the context of RF ablation and cryoablation. Interestingly, apart from wider lesions observed after ablation with the cryoballoon compared with point-by-point RF ablation, both techniques result in quite similar LGE lesion characteristics.68,69
Non-invasive Confirmation of Durable PVI
Most importantly, LGE-MRI can non-invasively evaluate and confirm durable PVI and may thus replace invasive repeat procedures confirming PVI. Today, it is a common practice that symptomatic recurrences beyond the 3 months post-ablation blanking period almost automatically trigger a repeat procedure. However, to an increasing degree, all four PVs are found isolated in those repeat procedures. As ablation of extra-PV targets has failed to show benefit in large randomised trials, more and more often we may end up performing these highly invasive procedures only to confirm durable PVI; or even worse, investigators might feel obliged to ablate extra-PV targets to justify the invasive procedure. Against this background it is noteworthy that LGE-MRI has been shown to be capable of reliably confirming durable PVI with positive predictive values approaching 100%.64 This is consistent with our experience where a complete circumferential LGE lesion set practically rules out PV reconnection. Thus, in patients with circumferential LGE lesions indicating durable PVI, there is no rationale for a repeat procedure, unless one is determined to target extra-PV structures (Figure 1).
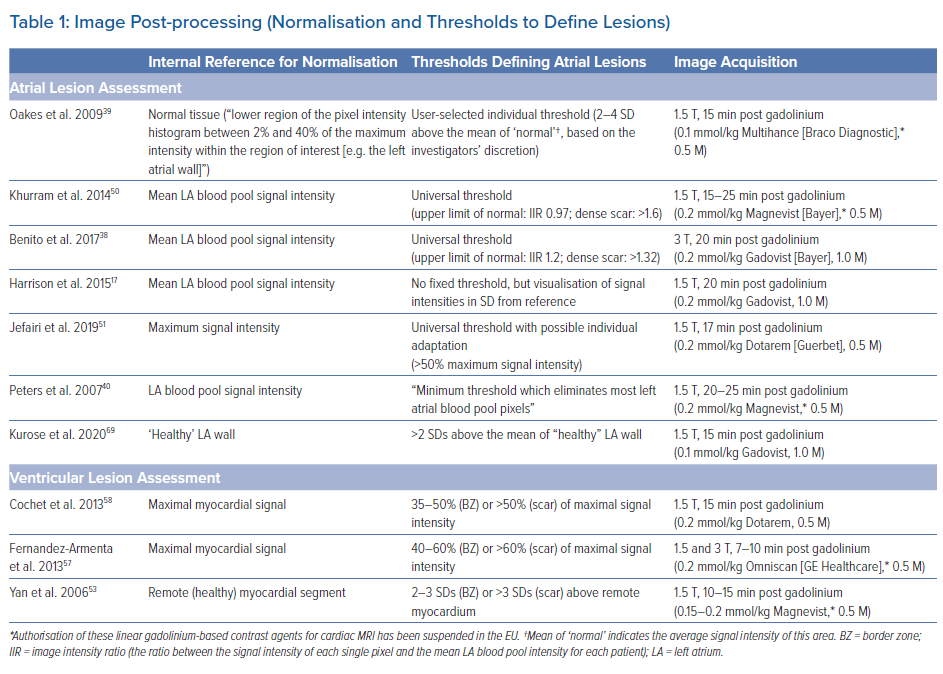
However, it should be noted that in earlier studies, complete LGE lesions encircling all four PV were encountered only in around 7–28% of the repeat procedures.64,70 Although we have to assume that these numbers have increased with recent advances in ablation techniques, in our experience the majority of patients still display discontinuities in the LGE lesions (Figure 2).
LGE-MRI-guided Repeat Ablation
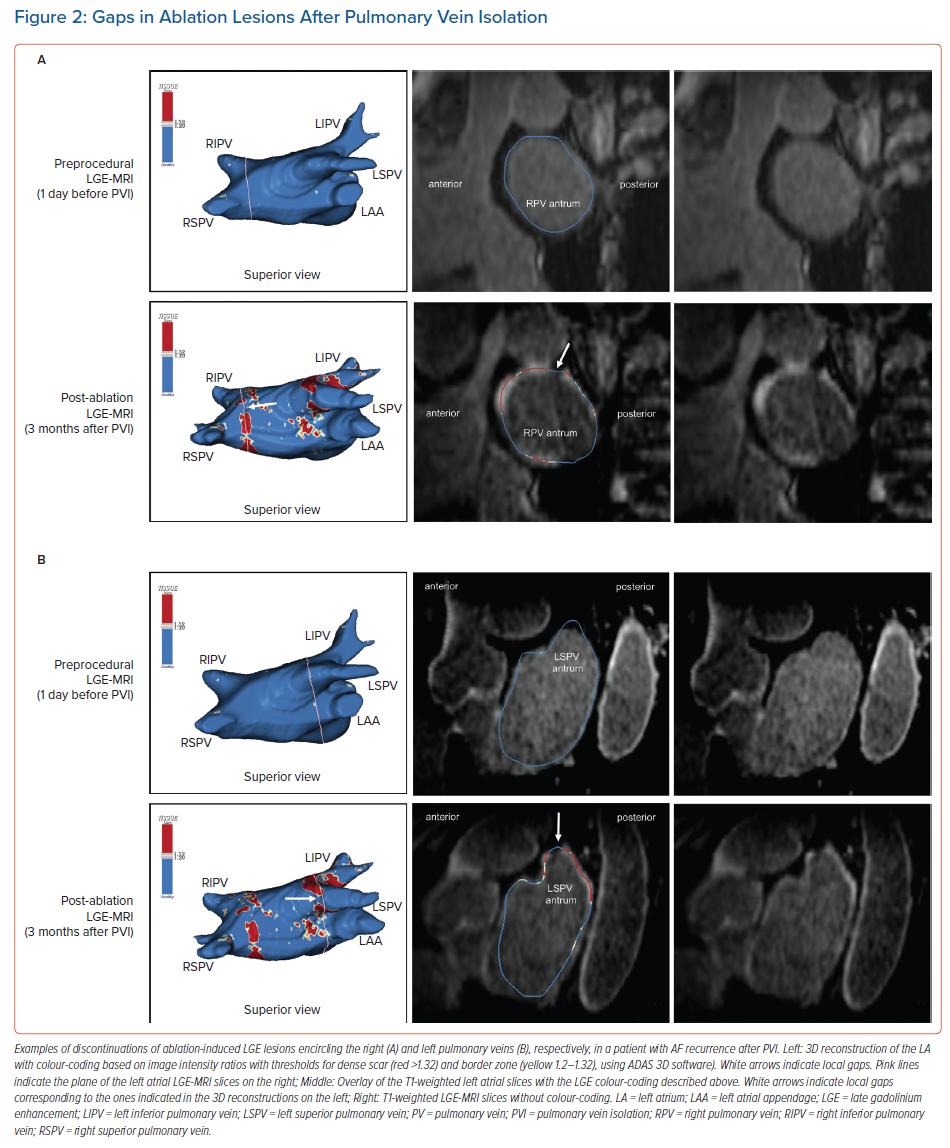
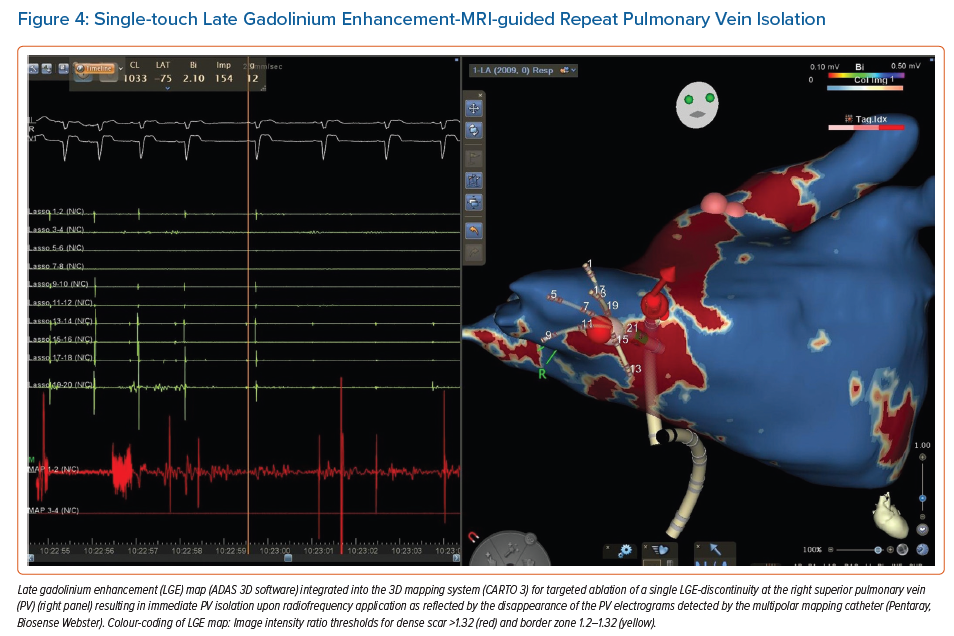
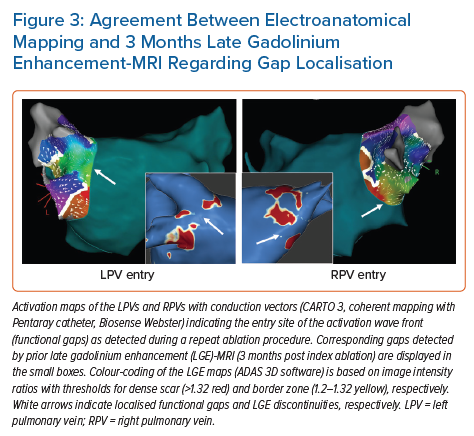
Accumulating evidence indicates that LGE-MRI can detect and localise the gaps in ablation lesions with high accuracy (Figure 3).5–7,14 Overall, the accuracy and in particular the high sensitivity in the detection of gaps appears to be sufficient for LGE-MRI-guided repeat ablation – not only in the context of AF (Figure 4), but also with respect to post-ablation reentrant atrial arrhythmias (Figure 5).5,7,14
Bisbal et al. were the first to demonstrate the feasibility of a merely LGE-MRI-guided approach in repeat PVI procedures.5 They performed re-ablation based on a 3D reconstruction of the atrial LGE-MRI, which was integrated and merged into the EAM system, with the investigator blinded to any electrical information. A total of 15 patients underwent this LGE-MRI-guided approach, with re-isolation being accomplished in 95.6% of the reconnected PVs. In a subsequent study, the same approach even proved superior to segmental PV re-isolation based on electrical signals.7 However, it has to be taken into account that this was not a randomised trial, but a case–control study with all its potential bias and limitations.
One possible explanation of the putative superiority of the LGE-MRI-guided approach could be the higher sensitivity of LGE-MRI regarding the detection of gaps. As outlined above, the negative predictive value of LGE-MRI to rule out gaps is very high.20 However, gaps detected by LGE-MRI do not always correspond to a functional gap based on electrical signals. While this may reflect a limited specificity of LGE-MRI and failure to detect local ablation-induced scarring, it may in part be explained by a limited sensitivity of catheter-based gap detection, particularly when conventional catheters are used instead of microelectrode catheters. Moreover, LGE-MRI-determined anatomical gaps might colocalise with non-conductive tissue or a site of dormant conduction, rendering catheter-based gap detection impossible.
Taken together, the higher sensitivity of the LGE-MRI regarding the detection of gaps may be at the cost of specificity, but it is less likely that gaps are omitted. Thus, while segmental electrogram-guided repeat PVI might occasionally result in undertreatment, LGE-MRI potentially leads to a more complete re-ablation.
Incomplete lesion sets can also constitute an arrhythmogenic substrate for reentrant atrial tachycardias, which is more likely in the case of extensive ablation strategies. Fochler et al. recently showed that incomplete lesions and resulting isthmi can be detected by LGE-MRI. They found that a dechannelling approach targeting LGE-MRI-detected isthmi, analogously to VT substrate ablation strategies, may be feasible and appropriate as a standalone approach in patients with recurrent atrial tachycardias after initial AF ablation.14
Lesion Assessment to Predict Ablation Outcome
Besides being a valuable tool for patient selection and guidance of repeat ablation procedures, LGE-MRI-based lesion assessment has yielded several predictors of AF recurrence, with the most obvious one being related to PV reconnection. Interestingly, Linhart et al. found LGE-MRI-detected gaps to predict AF recurrences, but it was not the presence of gaps per se or the number of gaps that predicted recurrences, but the cumulative length of all gaps added together relative to the circumference of ipsilateral PVs. In the 94 patients included in their study, the risk of recurrence increased by 16% with every 10% gap length relative to the PV-encircling ablation line. So, while a single small gap detected by LGE-MRI may not be critical with respect to outcome, extensive or multiple gaps are critical.6
These data are in line with a study by Akoum et al. using LGE-MRI before and 3 months after AF ablation to evaluate ablation lesions and modification of potentially arrhythmogenic substrate.71 They also found the presence of LGE-MRI-detected gaps in PV encircling ablation lines per se not to be predictive of recurrences. However, besides LGE-MRI-detected baseline atrial fibrosis, they identified residual fibrosis, i.e. fibrotic area not homogenised by ablation, as a predictor of AF recurrence.
Recent work by Kamali et al. assessed ablation lesions and their potential barrier function for electrical propagation in persistent AF.72 They identified the atrial area available for AF to propagate, as determined by LGE-MRI, as a predictor of recurrence after catheter ablation. Interestingly, this variable predicted recurrences better than did established predictors such as LA volume or total atrial fibrosis.
Ventricular Ablation Lesions
Objective of Lesion Assessment in the Ventricle
Even though LGE-MRI was first established for ventricular tissue characterisation and is by now widely used as a clinical tool to guide VT ablation through detection of arrhythmogenic substrate, there has been less interest in ablation lesion assessment in the ventricle than the atrium. This may be because of the fact that ablation strategies, and thus requirements for non-invasive ablation lesion assessment, are fundamentally different in the ventricle compared to the atrium. While in the atrium continuity and transmurality of predefined lesion sets are assessed, in the ventricle the endpoint is rather elimination or modification of arrhythmogenic substrate. The capability of LGE-MRI to accurately localise arrhythmogenic substrate in terms of scar border zone and slow conduction channels in a 3D fashion is meanwhile well-established; this is also true for patients with implanted cardiac devices when employing specific wideband MRI sequences.4,49,56,57,73–75 However, the elimination of LGE-MRI-detected arrhythmogenic substrate as a potential endpoint of VT ablation has not been studied.
Feasibility of Ventricular Lesion Assessment
Several preclinical and few early observational clinical studies evaluated the feasibility of LGE-MRI for ventricular ablation lesion assessment. Most of these studies investigated lesion formation in the acute setting, at time points when reliable discrimination of irreversible lesions from transient oedema based on LGE is highly challenging, if not impossible.9,12,76–78 While oedema has been demonstrated to resolve within 1–2 weeks from ablation, formation of definite lesions appears to take up to 8 weeks.10,11,25 Of note, Yamashita et al. demonstrated strong correlation of depth and volume of LGE lesions with definite lesions as determined by gross pathology in a canine model 8 weeks after ablation.10
Studies in patients with idiopathic and ischaemic VT have demonstrated that these definite ablation lesions can be visualised by LGE-MRI even many months after the ablation.15,16
The Dark Core Phenomenon
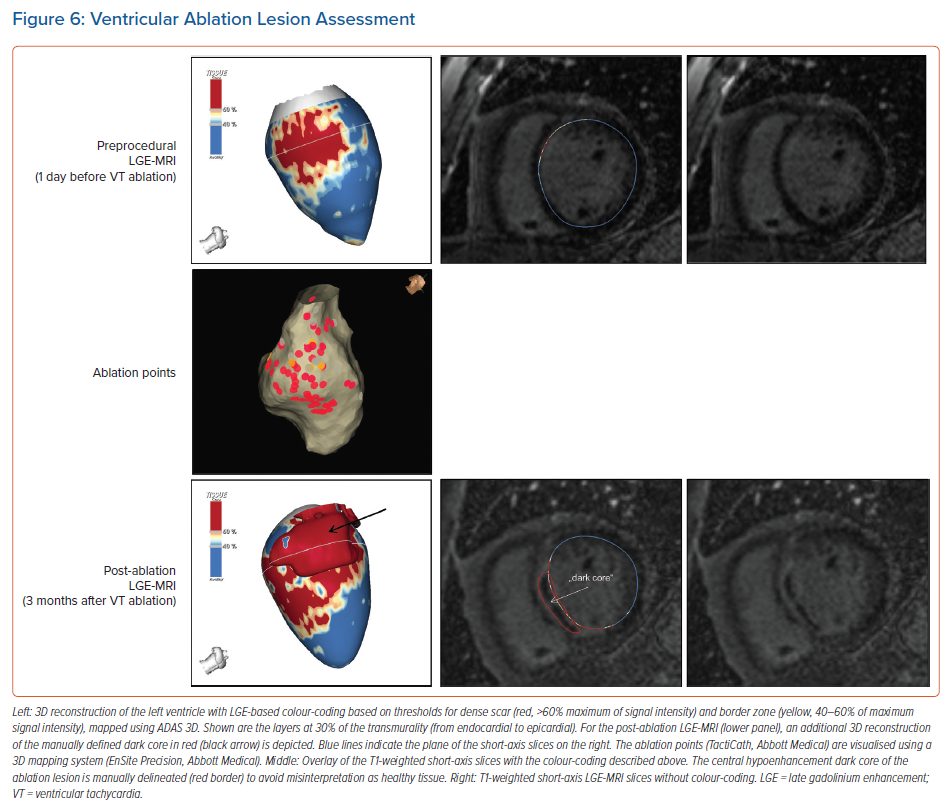
For image post-processing and analyses it has to be taken into account that, in contrast to atrial ablation lesions, the above-mentioned dark core phenomenon, characterised by centrally hypoenhanced lesions, has been observed even in these chronic stages of ventricular lesion formation, thus complicating the assessment of ablation-induced scarring in the ventricle.15 As current post-processing software algorithms are solely based on hyperenhancement, hypoenhanced lesion cores are not automatically identified and thus have to be delineated manually to avoid misinterpretation (Figure 6).
While Vunnam et al. have recently reported to have found ‘dark core’ lesions only up to 1 month after RF ablation but not at later stages, which is in line with previous preclinical and clinical data, Dabbagh et al. consistently observed lesions with hypoenhanced cores as late as 30 months post-ablation in all patients after repeat ablation of post-MI substrates.11,15,16,63 Of note, at our centre, we encounter centrally hypoenhanced lesions in around 60% of the patients at the systematic follow-up LGE-MRI 3–6 months post-VT ablation. Interestingly, in the study of Vunnam et al., comparison with pre-procedural LGE-MRI scans revealed that dark core lesions could only be observed in previously non-fibrotic myocardium without preexisting scar, suggesting that different wash-in/washout kinetics in scarred versus non-scarred myocardium play a role in this context. This is in line with a study in patients devoid of structural heart disease in whom LGE-MRI was performed at a mean of 22 months after ablation of idiopathic VT, where no central hypoenhancement of lesions was encountered.16
Potential Clinical Value of Ventricular Lesion Assessment
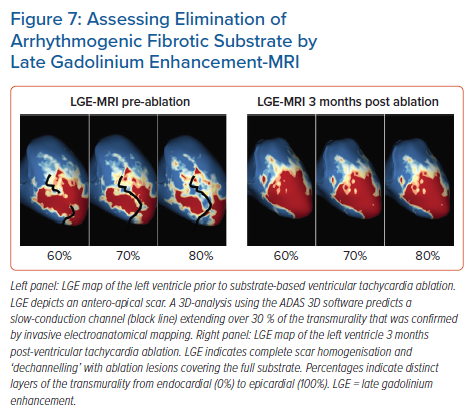
While cumulative evidence is suggesting feasibility of LGE-MRI-based ventricular ablation lesion assessment, clinical validation is absent. Against this background, we have recently analysed the potential role of LGE-MRI to assess the long-term effect of VT ablation in terms of arrhythmogenic substrate elimination (unpublished data). Three to 6 months following the procedure, effective ablation was reflected by pronounced reduction of LGE-MRI-detected border zone scar volume and extent of slow conduction channels compared to the preprocedural LGE-MRI (Figure 7). In patients undergoing repeat ablation procedures, this arrhythmogenic substrate elimination as determined by LGE-MRI correlated well with EAM. Thus, LGE-MRI-based lesion assessment may be of potential value to evaluate the efficacy of ventricular substrate ablation and to predict VT recurrences and clinical outcome. However, as mentioned above, clinical validation is warranted.
Conclusion
LGE-MRI constitutes the gold standard for non-invasive ablation lesion assessment. In the context of atrial ablation, LGE-MRI-based lesion assessment is already employed in routine clinical settings for non-invasive confirmation of durable PVI and to guide repeat ablation procedures in selected centres. In contrast, ventricular lesion assessment by LGE-MRI is less well established. However, despite the lack of clinical validation, LGE-MRI-based evaluation of arrhythmogenic substrate elimination holds great promise as an efficacy endpoint for VT ablation and a potential predictor of recurrences and clinical outcome.
In light of the current limitations, there is clearly some work ahead of us. Most importantly, uniform methodological and analytical standards are warranted to increase reproducibility of results across centres. This in turn, will foster the acceptance of the method and a broader implementation into clinical practice.
Clinical Perspective
- Late-gadolinium-enhancement (LGE)-MRI offers the unique capability of non-invasive ablation lesion assessment.
- LGE-MRI can detect and localise functional gaps in atrial lesion sets with high accuracy, which also allows for MRI-guided repeat ablation.
- Because of a very high negative predictive value regarding the detection of functional gaps, LGE-MRI can reliably rule out pulmonary vein reconnection non-invasively and may thus avoid unnecessary invasive repeat procedures where a pulmonary-vein-isolation-only approach is pursued.
- Elimination of LGE-MRI-detected arrhythmogenic substrate may serve as a potential efficacy endpoint and predictor of clinical outcomes in ventricular tachycardia ablation.