In clinical practice, atrioventricular (AV) junctional ablation is perceived as implementing damage to the AV conduction axis by delivering radiofrequency (RF) electromagnetic radiation to a site where it is possible to record an atrial deflection with the earliest His bundle potential, followed by the ventricular deflection from the right or left septum.1–7 Alternatively, alleged ‘specific’ ablation is achieved by simply targeting the site with the most prominent His bundle potential.5,8–20 In patients with AF, in particular, the recording of a His bundle potential is the only feature guiding the chosen site of ablation. Both approaches, however, are unlikely to deliver lesions at the location of the AV node or its extensions.21 A recent post-ablation autopsy study showed that even when the atrial signal is of the same size as the ventricular signal, and the His recording is early and stable, the AV node may not be affected by the procedure.7
Recent studies have shown that histologically specialised areas within the vestibules of the tricuspid and mitral valves, which form the atrial walls of the pyramid of Koch, expand to become the compact AV node, which then receives direct connections from the working myocardium of the atrial septum.22 Having been insulated by the fibrous tissue of the AV junction, the axis then becomes the non-branching AV bundle, with this component of the axis usually described as the His bundle. If, therefore, the recording is showing His bundle activation along with atrial and ventricular deflections, then the recording is being made at the site of the non-branching component of the conduction axis, subsequent to its insulation by the fibrous tissues of the AV junctions. The AV node itself, however, is positioned just inferior to the site of insulation of the conduction axis.21,22
Concerns have been expressed about the safety of the current approach, with instances reported of ventricular fibrillation, especially in patients with concomitant structural heart disease.23–27 In a meta-analysis that considered RF ablation aimed at producing complete heart block by excluding modification (as opposed to complete ablation) of the AV node, the procedure was associated with improved clinical outcomes. Sudden death, nonetheless, was reported in almost one-tenth of those included in studies with greater than 1 year of follow-up, and in up to just over one-twentieth for those followed for less than 1 year.28 The proarrhythmic potential of junctional ablation has been attributed to QT prolongation and increased QT dispersion in the early period after AV node ablation due to the abrupt drop in ventricular rate and change of ventricular activation, leading to short coupling interval ventricular extra beats that promote torsades de pointes.27,29 A faster post-ablation pacing rate at 90 BPM with gradual reduction has been reported to result in a significant reduction of the risk of sudden death (0.2%) compared with the risk at slower pacing rates of 60 BPM (2.1%).29 Notably, the mentioned meta-analysis included early studies that probably used pacing rates that were much slower than those adopted since the recognition of sudden death. In addition, these patients had a high prevalence of structural heart disease and heart failure, which are associated with an intrinsic risk of mortality. However, theoretically at least, the possibility of inappropriate creation of a proarrhythmic ventricular scar due to a large number of ablation lesions that are sometimes required to ablate the insulated His bundle cannot be excluded. Further, ablation of the His bundle, rather than the compact AV node, may reduce the possibility of a robust junctional escape rhythm. With all of these considerations in mind, we present here a method for anatomical ablation of the AV node itself, which potentially can avoid these complications.
Methods
Anatomical Position of the Atrioventricular Node
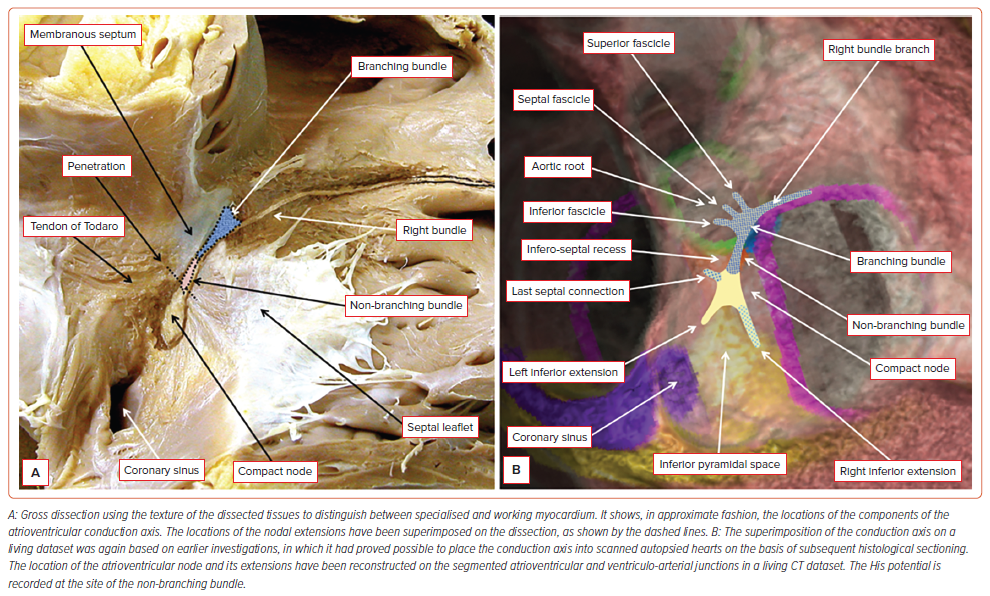
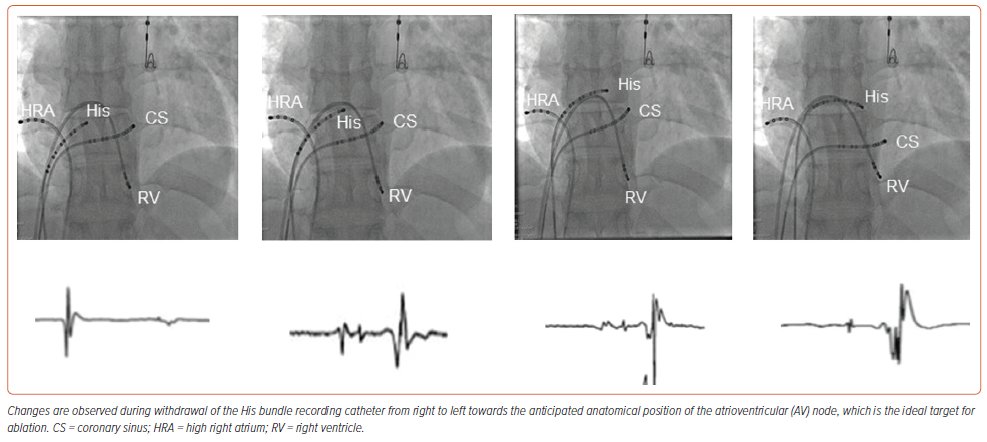
It is possible to show the approximate location of the AV conduction axis relative to the landmarks of the right side of the septal structures using gross dissection (Figure 1A). With this knowledge, it is then possible to superimpose the anticipated location of the axis on an image prepared using a living dataset obtained with CT (Figure 1B). In datasets from human hearts prepared with serial histological sectioning, the non-branching component of the conduction axis was shown to be 3.6±1.7 mm in length, varying from 1.7 to 7.2 mm.30 The distance between the compact node and the septal isthmus, using datasets also prepared by histological sectioning after 3D reconstruction, measured less than 4 cm (which was the calculated length of the course of the fast pathway during typical AV nodal tachycardia).31 The AV node is the proximal right atrial portion of the conduction axis that electrically connects the atria to the ventricles. The distinction between the node and the non-branching bundle is made at the point where the conduction axis itself becomes insulated by the fibrous tissues of the AV junctions from the working atrial myocardium. The node has a length varying between 5 and 7 mm, and a width between 1.0 and 1.5 mm.32 In anatomical terms, therefore, the distal extent of the node is posterior and inferior to the site of the catheter recording the His potential. This can be defined by withdrawing the His recording catheter back in a fashion that first records only the atrial and His potentials, and then fails to record a His potential (Figure 2). In this way, using the mapping catheter, it proves possible to identify the location of the AV node relative to the non-branching bundle.
Ablation Technique
Patients referred for AV junctional ablation underwent specific ablation of the AV node at the site as described above. If the patient was in AF, cardioversion was usually attempted. If this was not possible or desirable, then the patient was considered eligible for ablation only if a His potential could be recorded to allow implementation of the withdrawal technique. If the first RF application was ineffective, we usually continued delivery at the same site. There were occasions, however, when we had to reposition the catheter following de novo recording of the His. This was done at the operator’s discretion. In cases in which anatomic nodal ablation did not result in persistent AV block, ablation of the His bundle was performed. Following ablation permanent pacing was accomplished, taking care to start by programming a relatively high rate (>80 BPM).
Assessment of the Site of Block
A narrow QRS escape complex is most compatible with an AV nodal or early intra-His lesion in the absence of co-existent bundle branch block, and a wide QRS complex is most compatible with an infra-His problem.33 In addition, if conduction improves with atropine or exercise, or worsens with carotid sinus massage, the block is likely to be in the AV node. If block worsens with atropine or exercise, or improves with carotid sinus massage, it is probably infranodal.34 Assessment of block subsequent to seemingly successful ablation, therefore, was determined by the width of the escape rhythm. In select cases dependent on operator preference, assessment was based on a pharmacological challenge with atropine 10 minutes after creation of the lesion.
Procedural Endpoints
Primary endpoints were stable block without restoration of AV conduction subsequent to a waiting period of 20–30 minutes, with or without isoprenaline challenge, the presence of escape rhythm, and the QRS width of the escape rhythm. Secondary endpoints were the number of lesions required to produce block, and the procedure and fluoroscopy times.
The research reported in this paper adhered to CONSORT guidelines. The study received approval from each centre’s institutional review board.
Statistical Analysis
Descriptive statistics are summarised as means and standard deviations, or medians and IQR, as appropriate based on normality of distribution. Patient and procedural characteristics for patients with acutely successful anatomic AV node ablation were compared with those for patients in whom nodal ablation failed, and ablation of the His bundle was required. Data analysis was performed using Stata 16.1.
Results
Anatomical Nodal Ablation
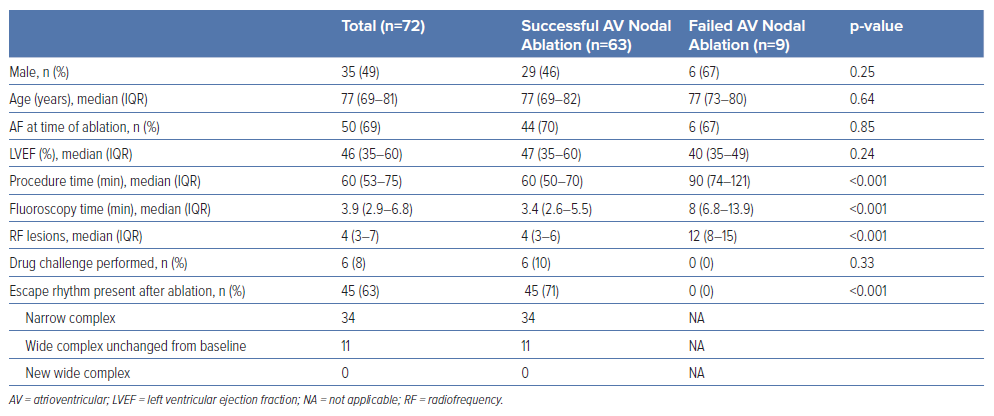
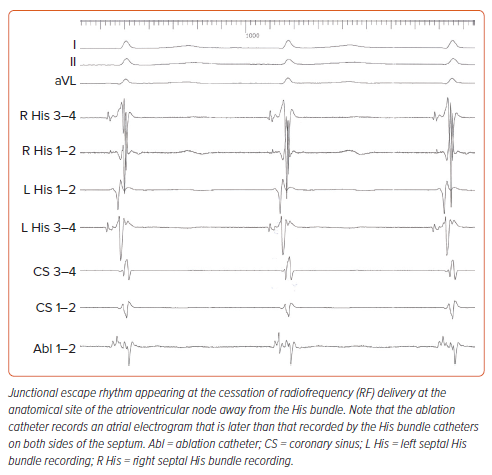
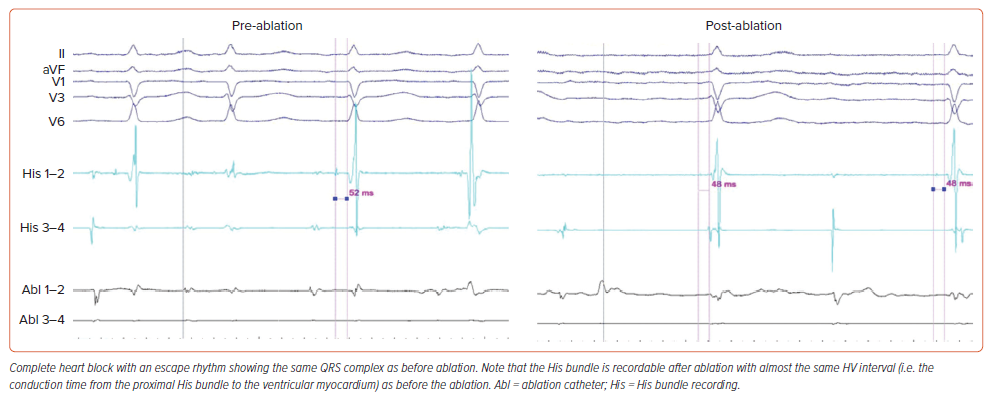
Anatomical ablation was attempted in 72 patients. Success was accomplished in 63 of them (87.5%), following 60 minutes (IQR 50–70 min) of procedure time, 3.4 minutes (IQR 2.4–5.5 min) of fluoroscopy time, and delivery of 4 (IQR 3–6) RF lesions. A non-irrigated ablation catheter was used in 63 patients, and an irrigated one in nine patients. Energy and temperature settings ranged from 35 W to 60 W and from 50°C to 70°C for non-irrigated catheters, and 30–35 W with a temperature limit of 43°C for irrigated ones. Electroanatomical mapping for positioning of catheters was used for nine patients. Data are listed in Table 1. At the site of successful ablation, a junctional rhythm with ventriculo-atrial conduction initially appeared (Figure 3). Continuation of delivery of RF energy eventually resulted in complete antegrade block (Figure 4). Junctional escape rhythm at a rate of 40–56 BPM, was present in 45 (71%) of successful ablations. The escape rhythm was narrow in all 34 patients with a narrow QRS complex prior to ablation, and similar to that before ablation in the remainder. Atropine was given to six patients after the 10-minute waiting period, and did not result in restoration of conduction. In four patients, atropine resulted in acceleration of the narrow escape rhythm.
In nine patients we were unable to interrupt AV conduction despite delivering 12 (IQR 8–15) lesions at the anticipated site of the AV node (Table 2). In these individuals we accomplished complete interruption of conduction by ablating at the site with a clear His bundle potential. Post-ablation escape rhythm was not present in any of the patients requiring His ablation (p<0.001 for comparison with the AV node ablation group).
Follow-up
During a period of follow-up varying from 3 to 32 months (median 10.5 months, IQR 5–14 months), seven patients died: six of non-cardiac causes and one due to intractable heart failure. We encountered no cases of sustained ventricular arrhythmias or sudden death. AV block had persisted in all patients at their last follow-up (Table 2).
Discussion
Our preliminary experience shows that ablation of the AV node itself is feasible and safe, and avoids the potential ventricular myocardial injury associated with extensive ablation of the non-branching bundle. The electrical activity of the node itself cannot be recorded in humans using conventional standard electrodes, despite initial reports of an extracellular nodal potential in animals and identification of the region of the AV node using microelectrode-embedded catheters that more accurately define the near-field compact AV node compared with conventional catheters.35–37 Only recently, Pandozi et al. recorded the nodal activity with the use of high-resolution electroanatomical mapping and specific filter settings.38 The difficulties in recording the nodal potential are related to the low mass of nodal tissue, slow conduction velocity causing lower amplitude electrograms, and the size and spacing of the catheters used for ablation. In addition, the node is relatively deep within the pyramid of Koch, being covered by a layer of working atrial myocardium. Thus, our technique, of necessity, was guided by anatomical landmarks.
Our results are in keeping with those reported by Berte et al. and indicate that induction of junctional ectopy denotes damage to the node and its extensions rather than to the His bundle.37 Intact retrograde ventriculo-atrial conduction during ablation most probably indicates interruption of the inferior extensions, whereas complete cessation of conduction is a marker of nodal damage.39,40 The possibility of modifying the node, as opposed to complete ablation, has also been raised. In our experience this approach is ineffective in providing long-term maintenance of control of the ventricular rate. Effective containment of AV conduction can be obtained only by complete ablation of the conduction axis. During follow-up, no cases of sustained ventricular arrhythmias or sudden cardiac death were recorded.
The main limitation of our method is that it may not be applicable to all cases. As judged by our criteria, we were unable to achieve interruption of the axis in one-eighth of our patients, meaning that we had to target the non-branching bundle. We do not know whether this can be attributed to existence of fibrous tissue, or the reported marked variability of the location of the conduction axis within the pyramid of Koch.41 In addition, the possibility that due to the availability of the conventional approach for ablating the His, our efforts towards elimination of the AV node were not sufficiently prolonged, cannot be ruled out. Theoretically, the systematic use of an irrigated catheter might have produced better results, but this assumption cannot be deduced from our data. In six of the individuals with failed anatomic nodal ablation, nonetheless, the procedure had to be guided in the presence of AF, without recording of distinct atrial activity. If, however, the His potential cannot be identified, our anatomical approach is not feasible. Second, the number of patients in the present study was too small to enable definite conclusions. In the current series, however, this approach appeared to be effective and safe over our follow-up period.
Conclusion
Ablation of the AV node using anatomical landmarks is feasible and safe. It results in an escape rhythm with a QRS similar to that recorded before ablation. It is theoretically superior to conventional His bundle ablation and may enable the emergence of a more robust junctional escape rhythm.
Clinical Perspective
- Ablation of the atrioventricular node using anatomical landmarks is feasible and safe.
- It should now replace the traditional His bundle ablation for interruption of the conduction system.