Thrombotic material in atrial fibrillation (AF) usually develops in the left atrial appendage as a result of decreased flow and stasis, possible endothelial dysfunction and a hypercoagulable state as indicated by increased fibrinogen, D-dimer, thromboglobulin and platelet factor 4 levels.1 In the Framingham Heart Study, the percentage of strokes attributable to AF increases steeply from 1.5 % in patients aged 50–59 years to 23.5 % in those aged 80–89 years.2 In patients with a history of hypertension but no prior diagnosis of clinical AF, subclinical AF predisposes them to embolic events.3 Undiagnosed silent AF is a probable cause of cryptogenic strokes,4,5 and subclinical episodes of AF are associated with silent cerebral infarcts, particularly in patients with diabetes.6,7
The frequency of AF-related incident ischaemic strokes in patients aged ≥80 years have increased threefold over the last 25 years, despite the introduction of anticoagulants, and are projected to futher increase threefold by 2050.8 Among patients with AF who are at moderate- to-high risk of stroke and are receiving anticoagulation, those with persistent AF have a higher risk of thromboembolic events and worse survival rates compared with those with paroxysmal AF.9 The risk of stroke is similar in patients with and without valvular disease.10 Serial ECGs, Holter monitoring mobile outpatient telemetry, external loop recorders and implantable loop recorders detect post-stroke AF in 23.7 % of patients.11 However, AF that occurs early after stroke can be caused by a transient neurogenic mechanism, and AF that occurs several months post-stroke can be an incidental finding; therefore, it cannot be concluded that the cause of cryptogenic stroke has been identified in all patients found to have post-stroke AF.11
Anticoagulation
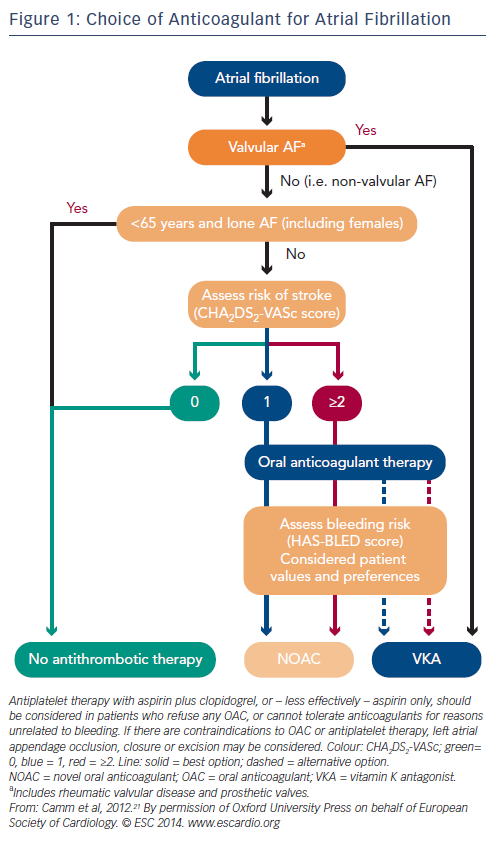
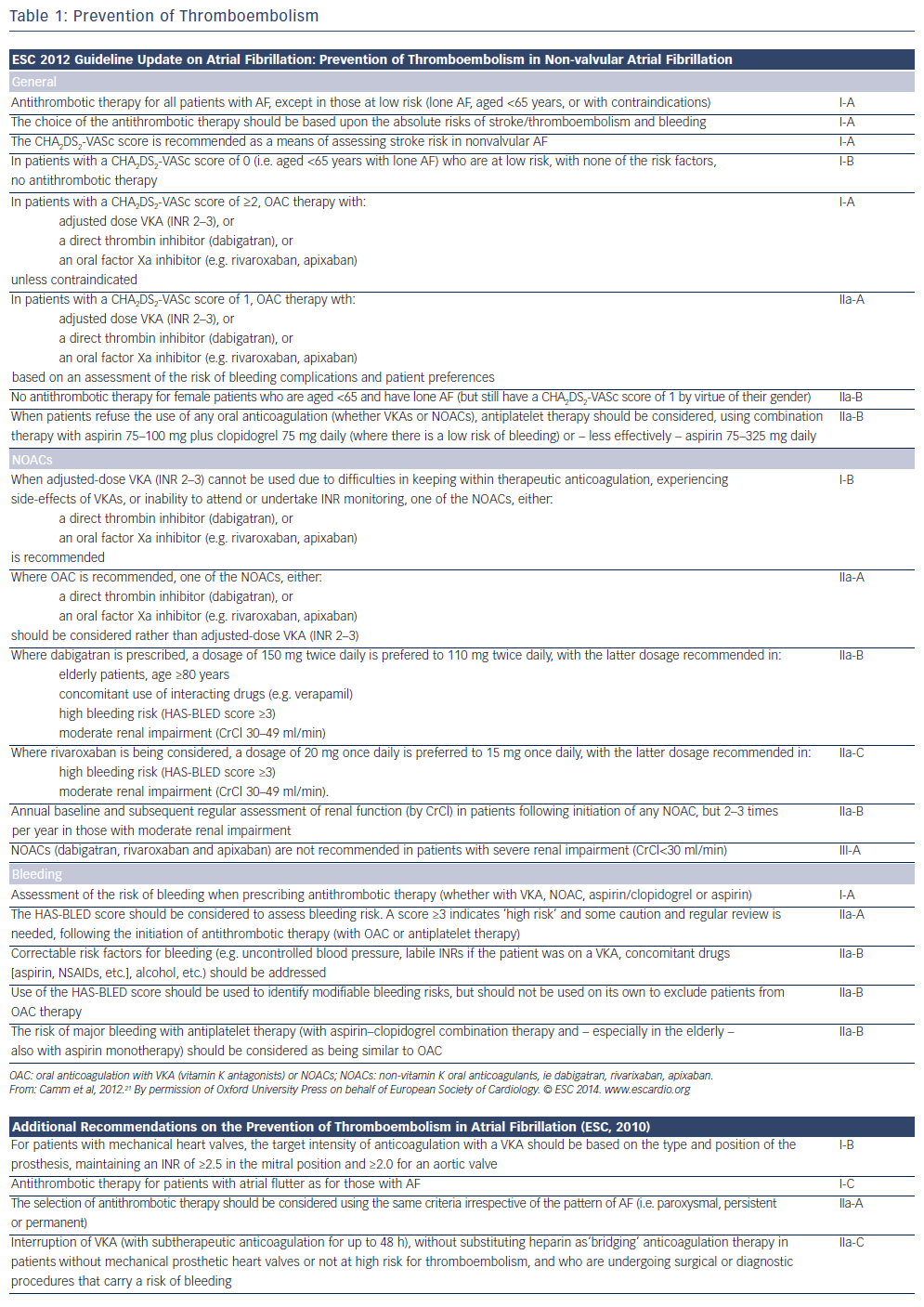
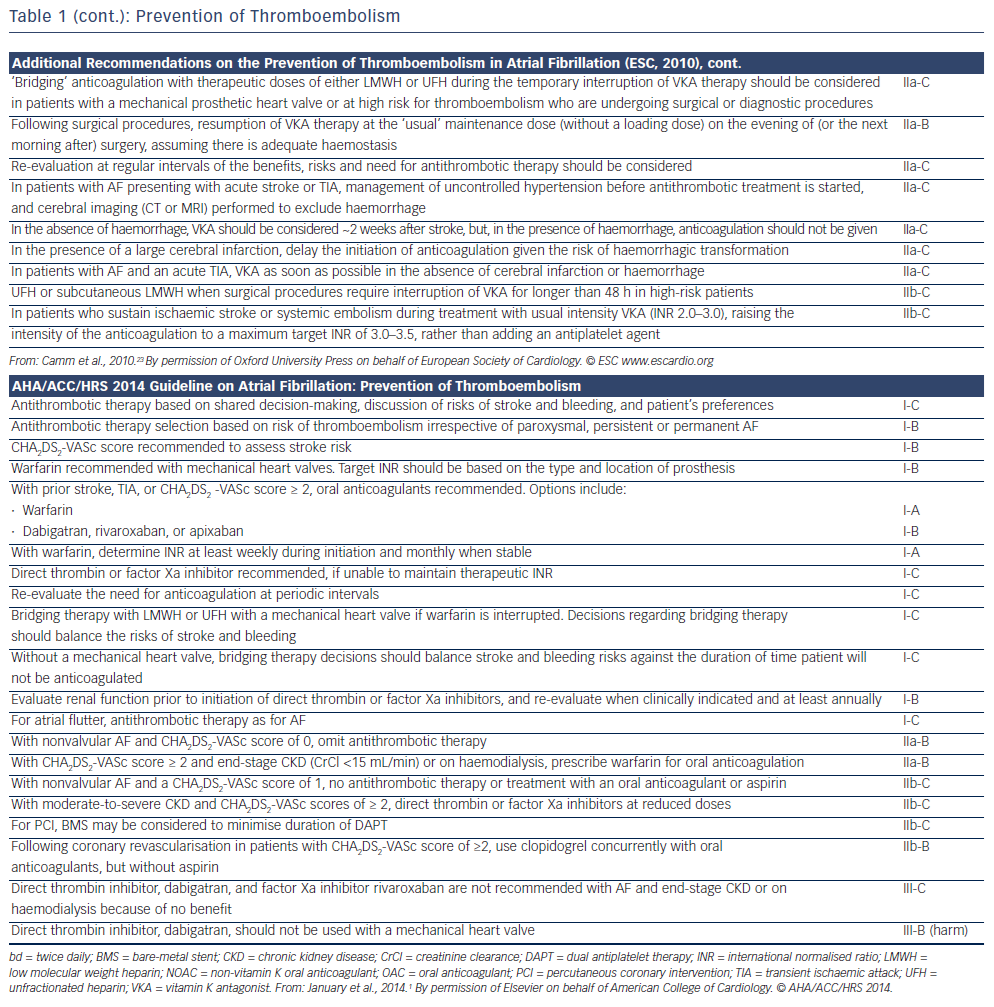
Adjusted-dose warfarin and antiplatelet agents reduce the risk of stroke by approximately 60 % and 20 %, respectively, in patients with AF.12 In general, oral anticoagulation is preferred in patients with CHA2DS2-VASc score ≥2, and no anticoagulation in patients with a score of 0 (see Figure 1 and Table 1). In patients with CHA2DS2-VASc score of 1, anticoagulation should be individualised as the risk of stroke is low.13 However, patients aged >65 years, especially women, are at high risk of ischaemic stroke,14 and in these individuals anticoagulation reduces the rate of mortality.15,16 The risk of bleeding is assessed by schemes such as the HAS-BLED, ATRIA and HEMORR2HAGES scoring systems.17 A HAS-BLED score ≥3 indicates ‘high risk’. New oral anticoagulants are now recommended for nonvalvular AF as a potential alternative to warfarin. Nonsteroidal anti-inflammatory drugs increase the risk of both serious bleeding and thromboembolism in anticoagulated patients with AF.18
Aspirin
The protective value of acetylsalicylic acid (aspirin) as monotherapy has come under question, and there are concerns that it may even increase risk of stroke in elderly patients (aged >75 years).19,20 Warfarin is superior to aspirin in patients aged >75 years, offering a 52 % reduction in yearly risk of a combined end-point of stroke, intracranial haemorrhage and peripheral embolism (1.8 % versus 3.8 %; Birmingham Atrial Fibrillation Treatment of the Aged Study [BAFTA]).15 Thus, the use of aspirin for stroke prevention in patients with AF should be limited to those who refuse any form of oral anticoagulation,21 or, perhaps, to those with a CHA2DS2-VASc score of 1.1
Aspirin and Clopidogrel
The combination of aspirin and clopidogrel offers increased protection compared with aspirin alone, albeit at an increased risk of major bleeding,22 and is preferred when warfarin is contraindicated. However, aspirin and clopidogrel together offer less protection than warfarin alone (RR of 1.44 for stroke, peripheral embolism, MI and vascular death).22 In patients who sustain an ischaemic stroke despite international normalised ratio (INR) of 2.0–3.0, targeting a higher INR should be considered (3.0–3.5) rather than adding an antiplatelet agent, as major bleeding risk starts at INR >3.5.23
Warfarin
Warfarin is a racemic mixture of isomers that inhibits the synthesis of vitamin K-dependent coagulation factors. The effective dose of warfarin varies significantly among individuals, as a result of genetic variations in its receptor, metabolism via the cytochrome P450 (CYP) system and interactions with other drugs, vitamins and green vegetables.1
The risk of AF increases with INR >3.5–4.0. Recommended INR values for AF are 2–3. Pharmacogenetic testing for guiding doses, by means of genotyping for the variants CYP2C9 and VKORG1, which are associated with reduced clearance and thus a decrease in warfarin requirement, is not clinically useful.24 Patients initiating warfarin may be at an increased risk of stroke during the first 30 days of treatment, probably owing to rapid deactivation of proteins S and C, two endogenous anticoagulants.25 In high-risk cases, warfarin should be started with concomitant low molecular weight heparin administration for the initial 3–5 days of treatment. Increased levels of coronary calcification have been recently reported in patients on long‑term therapy with vitamin K antagonists.26
Non-vitamin K Oral Anticoagulants
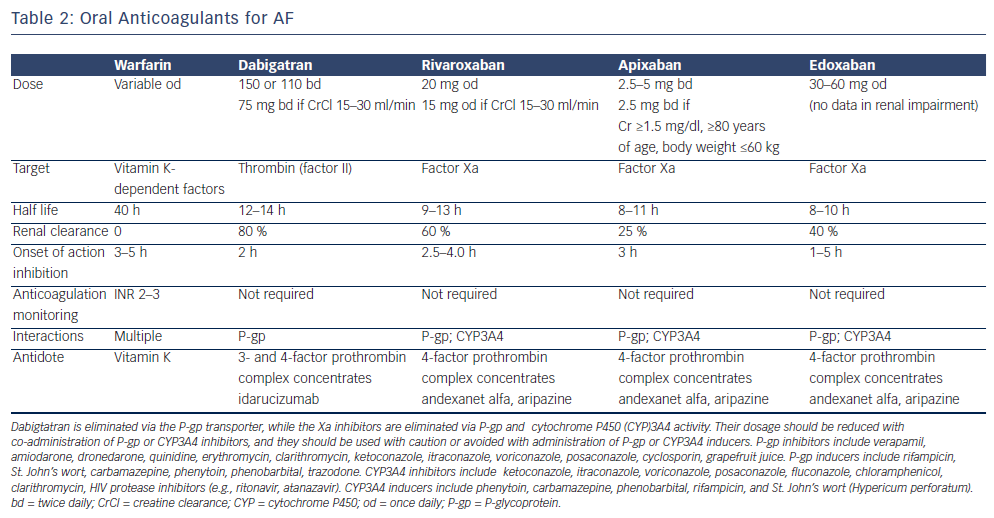
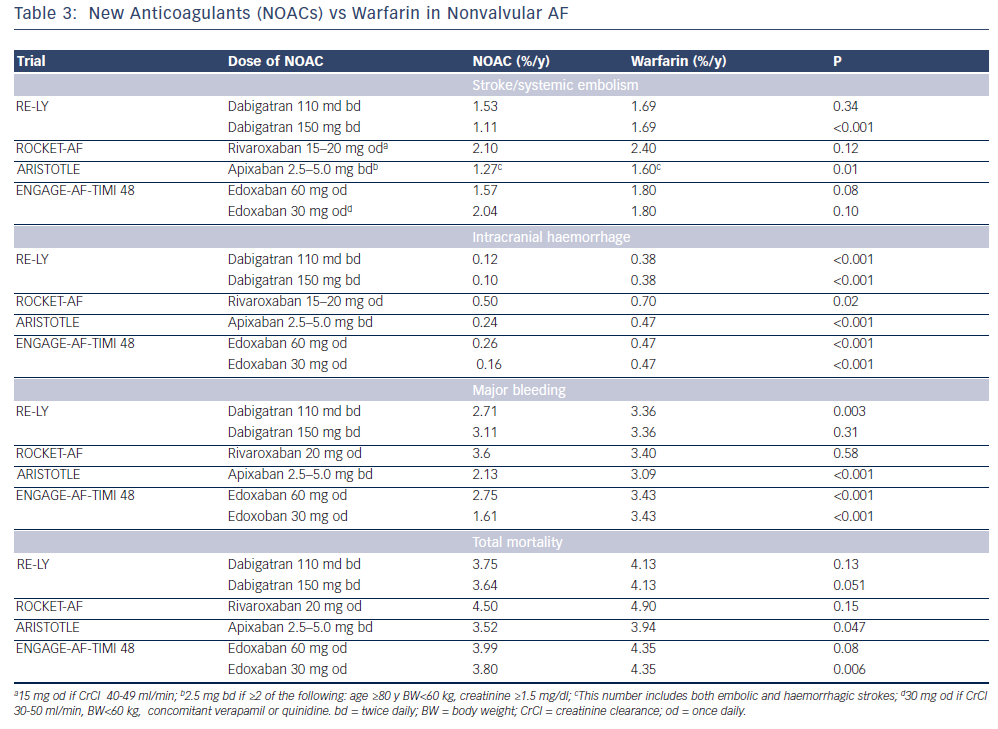
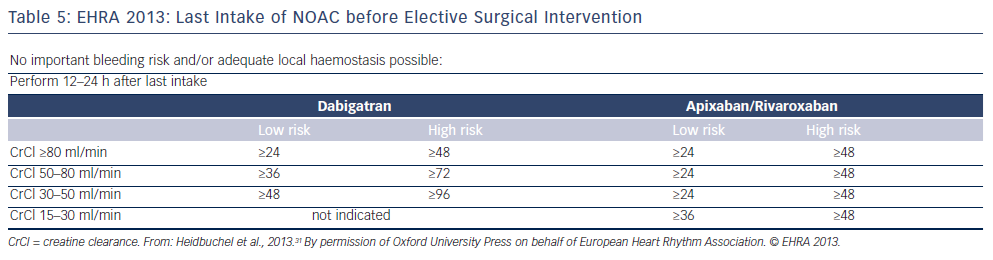
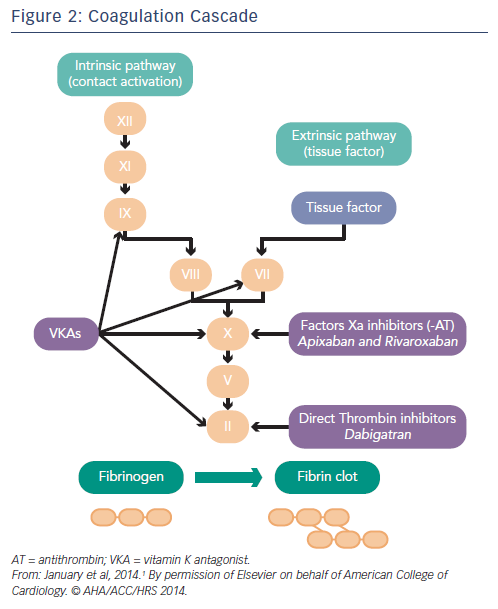
Non-vitamin K oral anticoagulants (NOACs) are direct thrombin (dabigatran) or factor Xa (rivaroxaban, apixaban, edoxaban) inhibitors. Thrombin catalyses the final step in the coagulation cascade by converting fibrinogen to fibrin. Factor Xa, in conjuction with factor Va, mediates activation of prothrombin to thrombin. In patients with non- valvular AF (ie not mechanical valves or mitral valve disease), they are associated with a relative 50 % reduction in the risk of intracranial haemorrhage and haemorrhagic stroke compared with warfarin that is also maintained in elderly patients. There is no need for frequent laboratory monitoring and dose adjustments.27,28 The main problems associated with NOACs are the lack of antidotes and specific assays to measure anticoagulant effect, and the considerably higher cost than warfarin.29 It should be also noted that all major clinical trials with warfarin have included patients without severe renal impairment (CrCl <25–30 ml/min), and renal function should always be considered, especially when treated with dabigatran (see Table 2). They are not indicated in patients on haemodialysis because they may precipitate inadvertent bleeding.30 NOACs do not interact with food but with inhibitors (or inducers) of P-glycoprotein transporters and CYP3A4. Caution is required when they are coadministered with drugs such as verapamil, amiodarone and dronedarone. In patients taking warfarin, switching to a new agent is appropriate when the INR is <2. The mode of action of novel oral anticoagulants in the coagulation cascade is presented in Figure 2. A comparison of new anticoagulants is presented in Tables 3 to 5. A practical guide by EHRA on the use of NOACs in patients with AF has been published (www.NOACforAF.eu).31
Direct Thrombin Inhibitors
Dabigatran
Dabigatran is preferred to warfarin for nonvalvular AF as recommended by the European Society of Cardiology21 and the Canadian Cardiovascular Society.32 In 2010, the US Food and Drug Administration (FDA) approved dabigatran at a dose of 150 mg twice daily (CrCl >30 ml/min), or 75 mg twice daily (CrCl 15–30 ml/min) based on the results of the Randomised Evaluation of Long-Term Anticoagulation Therapy (RE-LY) trial.33 However, in the Long-term Multicenter Extension of Dabigatran Treatment in Patients with Atrial Fibrillation (RELY-ABLE) trial, during 2.3 years of continued treatment with dabigatran, there was a higher rate of major bleeding with dabigatran 150 mg twice daily in comparison with 110 mg, and similar rates of stroke and death.34 The European Medicines Agency (EMA) has approved both the 110 mg twice-daily and 150 mg twice-daily doses for nonvalvular AF. Elective cardioversion may be performed in patients taking dabigatran for at least 3 weeks.21 Dabigatran is excreted through the kidneys and no dosing recommendation is given for clearance <15 ml/min. In elderly patients a reduced dose is reasonable (75 mg twice daily),35 especially for those aged >80 years. It can be used safely together with aspirin.33,36 A higher risk of major and gastrointestinal haemorrhage compared with warfarin has been seen in African Americans and patients with chronic kidney disease, but the risk of inrtracranial haemorrhage remains lower.37 Main side-effects of dabigatran are dyspepsia and stomach pain (11 %), and transaminase elevations 0.9–2.0 %, although with a frequency similar to that caused by warfarin. There is no evidence of liver toxicity as observed with ximelagatran.
A trend in the RE-LY study towards more MIs in the dabigatran arm as compared with warfarin was not confirmed in a subsequent post-hoc analysis.38 A recent meta-analysis of seven trials including the RE-LY detected a higher risk of MI (1.19 % versus 0.79 %; p=0.03),39 and this was also observed in the recent Secondary Prevention of Venous Thromboembolism (RE-MEDY) trial.40 However, in the recent Danish Registry report (4,978 patients on dabigatran and 8,936 patients on warfarin), rates of mortality, pulmonary embolism, and MI were lower with dabigatran compared with warfarin. Stroke/systemic embolism and major bleeding rates were similar in the two treatment groups.41 Thrombin time in diluted plasma (dilute TT using hemoclot direct thrombin inhibitor assay) and ecarin clotting time are precise methods to assess the anticoagulant effect of dabigatran. Activated partial thromboplastin time (aPTT) and prothrombin time (PT) are prolonged by dabigatran but the correlation is not linear to guide dosage.42 However, in the presence of a normal aPTT dabigatran is unlikely to contribute to bleeding, and aPTT can be used in emergencies as a rough estimate.43
Antidotes
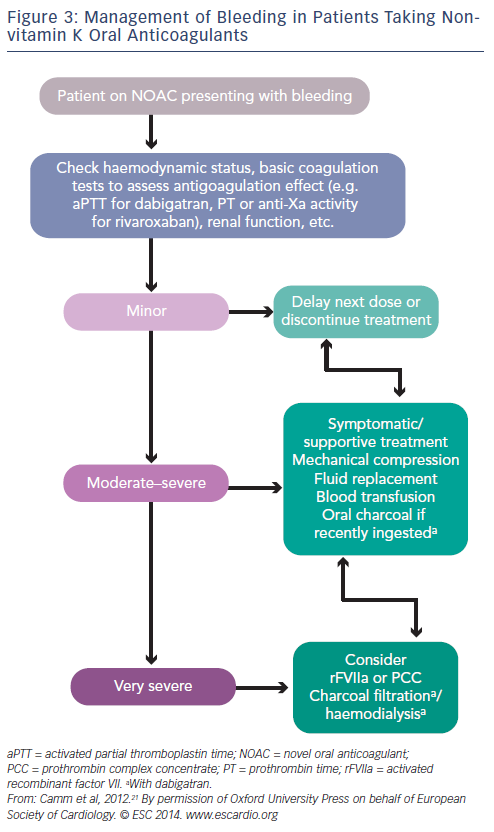
Fornonspecificandspecificantidotestodirectthrombininhibitors,please see the article by Rahmat and Lip, Monitoring the Effects and Antidotes of the Non-vitamin K Oral Anticoagulants, in this issue of the journal.
Factor Xa Inhibitors
Apixaban
Apixaban, an oral factor Xa inhibitor, is approved in Europe and Canada, and by the FDA for nonvalvular AF, and may be the most cost-effective NOAC.29,44 Apixaban has demonstrated reduced risk of stroke or systemic embolism without significantly increasing the risk of major bleeding or intracranial haemorrhage in patients with nonvalvular AF for whom vitamin K antagonist therapy was unsuitable (apixaban versus aspirin; AVERROES trial).45 In the Apixaban for the Prevention of Stroke in Subjects With Atrial Fibrillation (ARISTOTLE) trial, apixaban was found superior to warfarin in preventing embolic or haemorrhagic stroke, and resulted in less bleeding and lower mortality rates (11 % reduction; p=0.047).46 Benefits of apixaban have been seen in both paroxysmal and persistent/permanent AF.47 Rates of intracranial bleeding have been demonstrated to be significantly lower in patients treated with apixaban than with warfarin, regardless of renal function.48 Benefits of apixaban are irrespective of concomitant aspirin use49 or of patients’ age.50
A substudy of the ARISTOTLE trial has also shown that cardioversion of AF can be safely performed in apixaban-treated patients.51 The drug is metabolised in the liver via P450-dependent and -independent mechanisms and 25 % is excreted renally. It is not recommended for use in patients with severe hepatic impairment. Apixaban is also not recommended in patients receiving concomitant treatment with strong inhibitors of both CYP3A4 and P-glycoprotein, such as azole antimycotics and HIV protease inhibitors, and should be used with caution in patients taking rifampicin, phenytoin, carbamazepine and phenobarbital. There are limited clinical data on patients with a CrCl of 15–29 ml/min, and the drug is not recommended in patients with a CrCl of <15 ml/min. Anti- factor Xa assays may be used to estimate the anticoagulant effect. APTT and PT are prolonged by apixaban but they cannot be used to guide dosage as the correlation is not linear, especially with PT.42 Concomitant use with diltiazem results in increased apixaban levels.
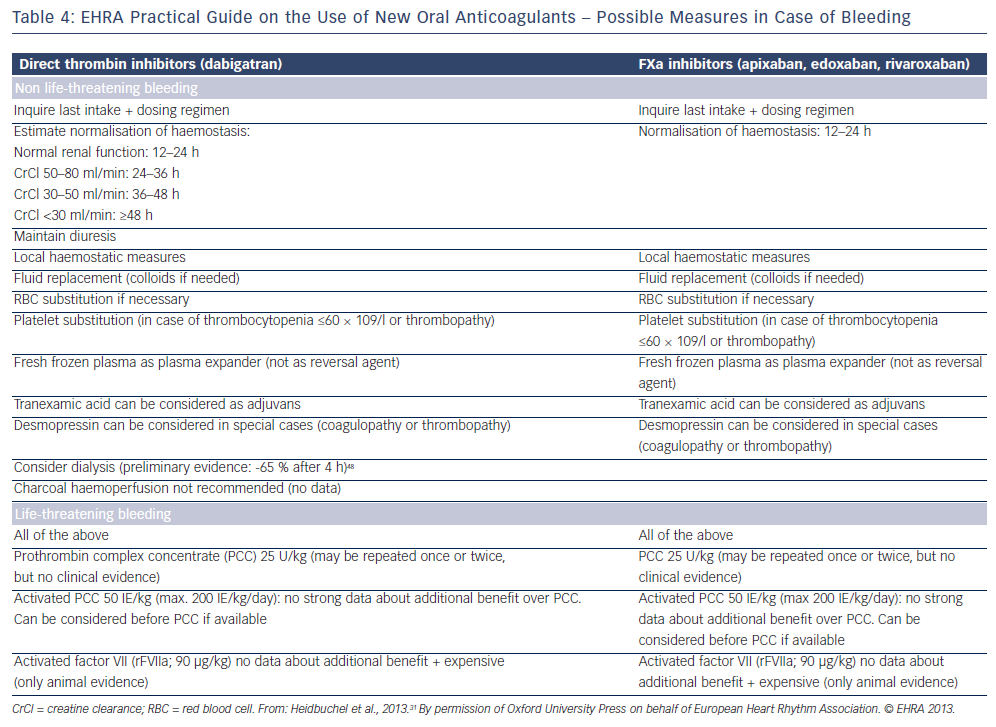
Rivaroxaban
Rivaroxaban is an oral factor Xa inhibitor that has been approved by the FDA and EMA for nonvalvular AF. In the ROCKET-AF trial (Efficacy and Safety Study of Rivaroxaban With Warfarin for the Prevention of Stroke and Non-Central Nervous System Systemic Embolism in Patients With Non-Valvular Atrial Fibrillation), rivaroxaban was not found to be inferior to warfarin (INR 2–3) in patients with nonvalvular AF for the prevention of stroke or systemic embolism, and offered a lower rate of intracranial bleeding, but a higher rate of gastrointestinal bleeding.52 In a substudy of this trial, rivaroxaban demonstrated equal safety and efficacy with warfarin in patients aged >75 years.53 The half-life of rivaroxaban is 7–11 hours, but factor Xa is inhibited for up to 24 hours, allowing once-daily dosage. Its bioavailability increases with food consumption. The drug is metabolised in the liver via P450- dependent and -independent mechanisms, and is not recommended in patients receiving concomitant treatment with strong inhibitors of both CYP3A4 and P-glycoprotein inhibitors (see the apixaban section). The drug is not recommended in patients with a CrCl of <15 ml/min. Anti-factor Xa assays are used to estimate the anticoagulant effect.21 APTT and PT are prolonged by rivaroxaban but they cannot be used to guide dosage as the correlation is not linear.42 However, prolonged PT bleeding can be attributed to rivaroxaban, and PT can be used as a rough estimate in case of emergencies.43
Edoxaban
Edoxaban has been demonstrated as noninferior to warfarin with respect to prevention of stroke or systemic embolism and shown to be associated with significantly lower rates of bleeding and death from cardiovascular causes (ENGAGE AF-TIMI 48 trial), and it is approved by the FDA.54 Both the 30 and 60 mg doses were not inferior to warfarin, but in the intention-to- treat analysis with the 60 mg dose there was a trend favoring edoxaban.
Betrixaban
Betrixaban has also been found equivalent to warfarin.55
Antidotes
For nonspecific and specific antidotes to factor Xa inhibitors, please see the article by Rahmat and Lip, Monitoring the Effects and Antidotes of the Non-vitamin K Oral Anticoagulants, in this issue of the journal. Although clinical experience is limited, antidotes for Xa inhibitors should be effective for all available agents.
Transition between NOACs/Warfarin
Transitioning from one anticoagulant to another is a period of high risk for both strokes and bleeding. A reasonable strategy is to reduce the NOAC dose by half, start warfarin and stop the NOAC when the INR is ≥2. When the patient is on warfarin, the NOAC is started after cessation of therapy and three-daily INR measurements to detect a value <2.56
Conclusion
In conclusion, the new, non-vitamin K dependent oral anticoagulants, ie direct thrombin or Xa inhibitors, appear to be safer and more effective than warfarin in preventing thromboembolism in patients with non-valvular AF (ie absence of prosthetic valves or rheumatic mitral valve disease).