Ventricular arrhythmias are designated idiopathic when demonstrable structural heart disease, significant coronary disease including coronary spasm or genetic arrhythmia syndromes are absent.1 These arrhythmias may be benign but are also a recognised cause of sudden cardiac death. The common form of idiopathic ventricular tachycardia (VT) originates in the ventricular outflow tracts, manifest with extra-systoles with long coupling intervals or runs of repetitive VT, and is generally not associated with increased mortality.2 However, more malignant ventricular arrhythmias may also be idiopathic, but frequently originate with short-coupled ventricular extra-systoles that result in polymorphic VT and may degenerate into VF.3,4,5
Idiopathic ventricular arrhythmias may have variable sites of origin. In addition to the common sites of origin from the right and left ventricular outflow tracts, the fascicles of the conduction tissue, perivalvular tissue and papillary muscles are other sources of origin.6 Less often they originate from the epicardium, free-running Purkinje fibres and intra-cavitary structures such as the moderator band (MB).7 Short-coupled premature ventricular contractions (PVCs) originating within the Purkinje network specifically have been identified as a source of triggered malignant VF.4,5,8 Haïssaguerre et al. described VF initiated by triggers within the Purkinje system.4,5 Nakamura et al. identified PVC triggers in thirteen patients presenting with VF without structural heart disease, four of whom had origination from Purkinje potentials or MB. Ablation of the triggering PVC was successful in suppressing recurrence of VF.9 Many primary VF events were initially regarded idiopathic until specific phenotypic and genotypic patterns eventually became evident to classify them as disease entities with distinct pathophysiological mechanisms (e.g. Brugada syndrome, early repolarisation syndrome). Until the use of intracardiac echocardiography (ICE) became common place in VT ablations, the MB as a source of short coupled PVCs and VF was not well recognised 7,10–12 The MB is a right ventricular structure composed of Purkinje and myocardial tissue and an important target for potentially curative ablation, but ablation at this location has certain important considerations. The aetiology, diagnosis and management of arrhythmias originating from the MB are reviewed herein.
Anatomy and Electrophysiology of the Moderator Band
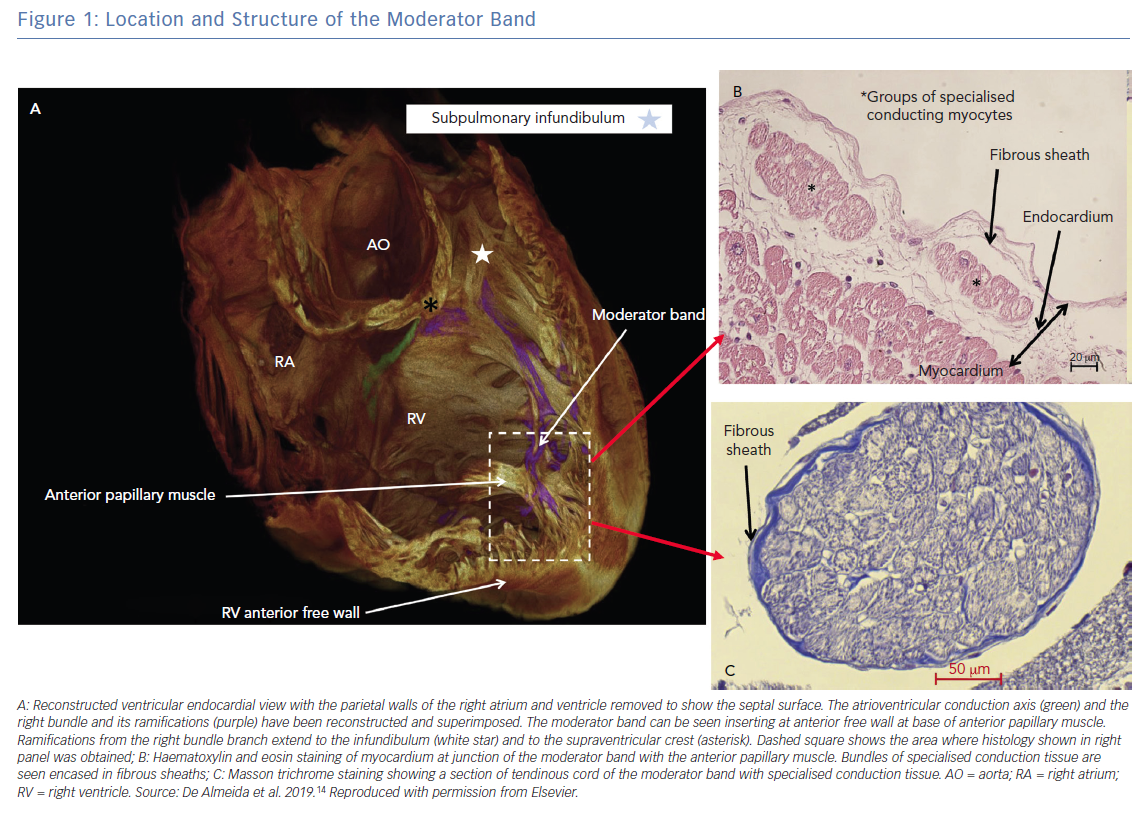
The MB is present, with varying prominence, in all human hearts and in other primates. It is an intra-cavitary structure in the right ventricle (RV) spanning from the lower limit of the inflow tract of the RV anterior septum to the base of the anterior papillary muscle of the RV free wall. It is part of the septo-marginal trabeculation that provides support to the anterior papillary muscle of the tricuspid valve (Figure 1).13 Its name originates from a traditional description that the structure ‘moderated’ the RV by preventing excessive distention. In addition to this postulated function, it is now known that it carries within it a fascicle of the right bundle that allows for rapid activation of the RV free wall (Figure 1).14 Transection of the MB often results in a distal right bundle branch block pattern. The morphology of the MB is highly variable, ranging from short and thick (the commonest variant) to long narrow bands of muscle. Additionally, its location along the length of the RV from the tricuspid valve to the apex is variable, with the majority being in the apical half of the RV cavity. Vascular supply to the MB originates from the anterior interventricular branch of the left coronary artery with anastomotic contribution from the right coronary artery at the base of the RV anterior papillary muscle, which forms an important collateral circulation between the left and right coronary arteries.13
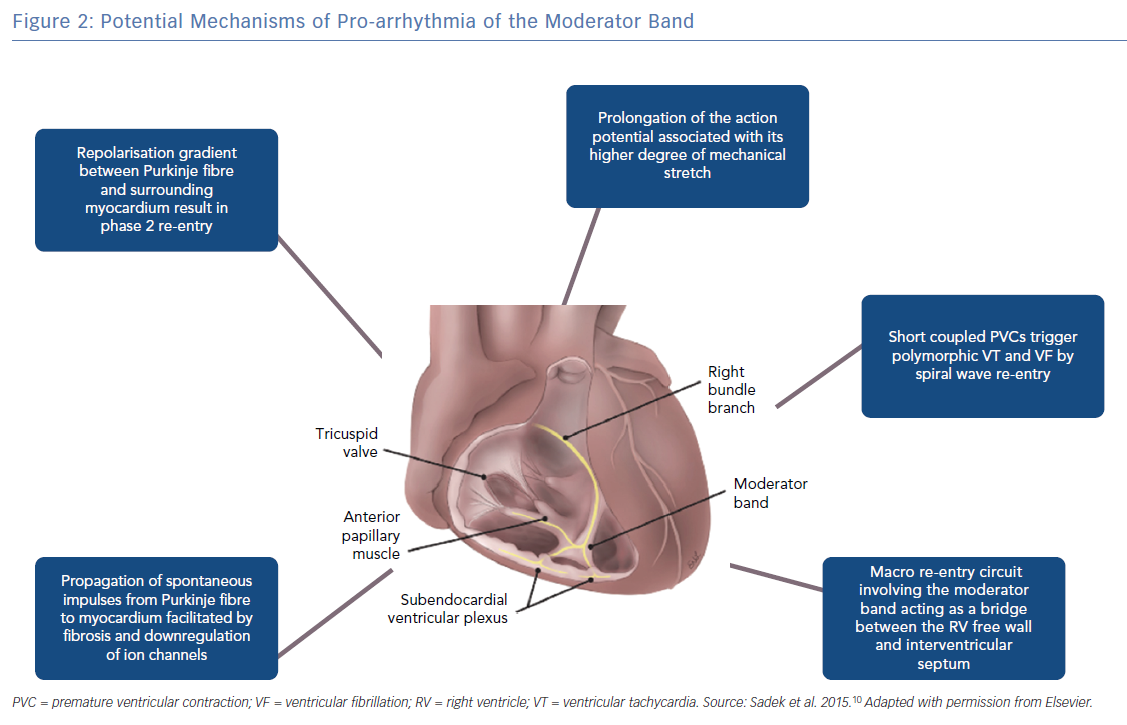
Structurally the MB is highly organised, composed of rapidly conducting specialised Purkinje fibres surrounded by ventricular myocytes.14,15 The Purkinje fibres are insulated from surrounding ventricular myocardium by collagen and adipose tissue until peripheral arborisation along the RV-free wall (Figure 1).14,16 There is considerable inter-individual variability in thickness and the ratio of muscle to Purkinje fibre that is likely a determinant of the proarrhythmic potential of the MB.15 There are multiple mechanisms by which the MB participates in arrhythmias (Figure 2).16
In most ventricular arrhythmias implicating the MB, Purkinje potentials are often demonstrable preceding the onset of arrhythmia and dissociation or abolition of the potential is a marker for successful suppression of arrhythmias.4,10,11 Purkinje fibres have been shown to initiate arrhythmias by several mechanisms including enhanced automaticity, triggered activity and re-entry.16 In the genesis of PVCs, triggered activity is the likely mechanism. Afterdepolarisations can be initiated by traveling Ca++ waves and alteration in balance of inward and outward currents.16 Ca++ waves can initiate membrane depolarisation in a well polarised aggregate of Purkinje cells in the absence of a specific stimulus.17 In addition, the transient outward current (Ito) has been implicated in Purkinje early afterdepolarisation.18 Genome-wide haplotype-sharing analysis of Dutch families with idiopathic VF identified over-expression of the DPP6 gene. Upregulation of DPP6 in the Purkinje fibre was subsequently shown to enhance Ito in cardiac Purkinje fibres relative to ventricular muscle. This strong repolarisation gradient between the Purkinje fibre and the surrounding ventricular myocardium can result in short-coupled PVCs possibly because of phase 2 re-entry.19
The mechanism for conduction and perpetuation of an afterdepolarisation from the Purkinje fibres to the surrounding myocyte is a matter of debate as the Purkinje fibre source is small compared to the larger myocardial sink. In a computational modelling study by Xie et al., the presence of fibrosis or downregulation of IK1 and ICaL, lowered the number of source cells required for early or delayed afterdepolarisation induced triggered action potentials.20 This may explain why some patients develop arrhythmias related to Purkinje firing while others do not. Once repetitive stimulation occurs, sustained ventricular arrhythmias can be due to re-entry or spiral wave break up into fibrillation. In the sheep and human wedge preparations Walton et al. demonstrated shorter refractory periods in the Purkinje fibres of the MB compared to surrounding myocardium and were able to demonstrate macro-re-entry between the RV free wall and the interventricular septum.15 Whether such a macro-re-entrant mechanism is operative in the clinical arrhythmias involving the MB is unclear. In the series by Sadek et al., one patient has recurrent monomorphic VT triggering ICD shocks and that was ablated successfully in region between the MB and free wall insertion.10
The MB is also subject to stretch-related arrhythmia because of its position spanning the septum and RV free wall. Mechanical stimulation of the ventricular myocardium is known to generate membrane depolarisation, prolongation of action potential duration and triggered activity.21
A more benign arrhythmia related to the MB is via atrio-fascicular accessory pathways that are known to insert into the Purkinje system of the MB making it part of atrioventricular reciprocating tachycardias in patients with the Mahaim accessory pathway.13 Although the MB might be part of the circuit, ablation for a Mahaim tachycardia is usually targeted at the atrio-fascicular pathway adjacent to the tricuspid annulus.
Clinical features of Patients with Moderator Band-related Arrhythmias
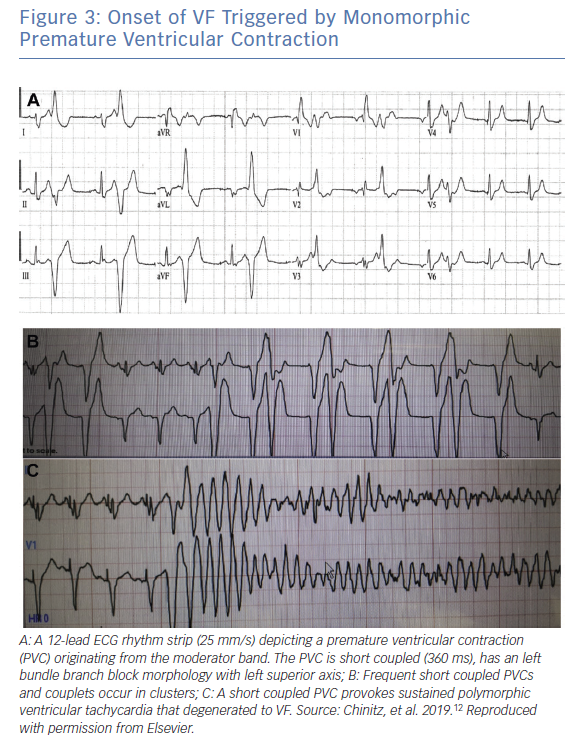
The arrhythmias of MB origin described in the literature range from monomorphic PVCs to VF with electrical storm.7,10–12 There is little information on the MB related PVC or repetitive VTs. Clinical series and case reports provide more information on patients who present with idiopathic VF, usually provoked by monomorphic PVCs. The patients who manifest VF often present with cardiac arrest as their initial symptom and frequently have electrical storm. The mean age of patients in the published series is the mid-40s (range 27–61 years) with probable higher propensity in males.7,10–12 Cardiac testing including coronary angiography, transthoracic echocardiography and contrast enhanced cardiac MRI are negative for structural heart disease. All episodes of VF in the hitherto published reports appear to be triggered by short coupled PVCs with coupling interval less than 400 ms. In some cases, an underlying bradycardia and pause dependency is present.7 Arrhythmias tend to occur in clusters with bigeminal PVCs that may lead to triggering of VF.
Electrocardiographic Diagnosis
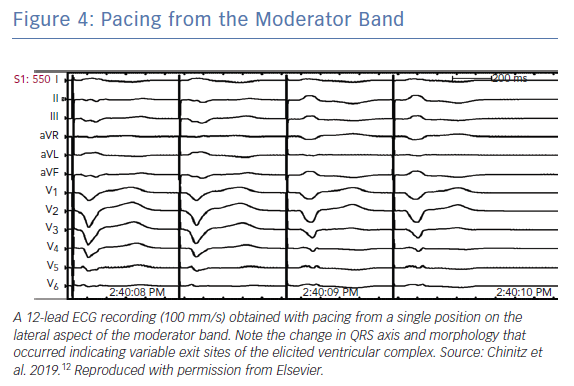
Consistent with their origin from within the apical RV, PVCs from the MB typically have a left bundle branch block (LBBB) pattern and a left superior axis with positive QRS in leads I and aVL (Figure 3).10 Sadek et al. described the PVCs as being relatively narrow with a mean QRS duration of 152.77 ± 15.2 ms with an intrinscoid deflection in the precordial leads <100 ms.10 Precordial QRS transition is typically later than V4 and always later than that of the sinus QRS in keeping with an apical lateral origin of the PVC. However, ectopy may exit the MB from its insertion points, predominantly at the septum or in the anterior papillary muscle, and may therefore result in variable precordial transition and frontal plane axis (Figure 4).22 This variability may complicate differentiation of PVCs originating from the MB from those originating from other RV regions.
Management of Moderator Band-related Arrhythmias
The exact incidence of PVCs or monomorphic VT of MB origin is unknown as early reports of ablation for these benign arrhythmias rarely used intracardiac echocardiography to define exact sources. Most recent reports of MB related arrhythmias refer to PVC-mediated VF. There are very little data on the medical therapy of VF triggered by MB PVCs but as the Purkinje fibres are the likely trigger for the arrhythmia, acute medical therapy for VF suppression appear to follow the general principles of management of idiopathic VT. In one of the early reports of MB-triggered VF, pause dependency was evident and atrial pacing was effective for acute suppression.7 The use of isoproterenol that is highly effective for suppression of electrical storm in the early repolarisation syndrome23, 24 has not been reported in MB related VF but is a consideration when conventional drugs such as lidocaine, procainamide or amiodarone fail to control the arrhythmia.
The ability to suppress the Ito with quinidine, disopyramide and bepridil has proven useful for suppression of idiopathic VF. Belhassen et al. reported effective chronic suppression of inducible VT and VF in patients with idiopathic VF treated with high doses of quinidine (1,000 to 2,000 mg/day).25 However, quinidine in such high doses is seldom well tolerated and the drug is no longer widely available. Its effect on PVC triggered VF of MB origin is not well described but therapy with quinidine is worth attempting in cases where conventional antiarrhythmics prove ineffective. It should be borne in mind that quinidine has the potential for QT prolongation and torsade de pointes VT in susceptible patients.
Ablation of Moderator Band-associated Arrhythmias
Several case reports and case series indicate that ablation of ventricular arrhythmias originating from the MB is an effective form of therapy especially in PVC-mediated VF and electrical storm.7,10–12 Early reports of successful ablation of idiopathic VF targeting Purkinje mediated PVCs must have included the MB but not recognised as such because ICE was not in common use. In one multicentre study of 27 patients with idiopathic PVC, the Purkinje system in the anterior RV was involved in nine of them.4 At least some of these were likely of MB origin based on ECG examples in the report. In this series, ablation was successful in suppressing recurrent VF in the majority (89%) with some of the patients able to avoid a defibrillator implant after prolonged in-hospital monitoring showed no further arrhythmia.4 In the series by Sadek et al., 10 of 394 patients with idiopathic ventricular arrhythmias had an MB origin.10 Seven of these presented with VF. Ablation was performed targeting Purkinje potential either at the septal or free wall insertion and guided by intracardiac echocardiography. Six of the 10 patients needed a second ablation (three had already undergone prior ablation attempts). Arrhythmia suppression was achieved without drug use in nine of 10 patients during a mean follow up of 21 months.10 These reports confirm the efficacy of ablation for Purkinje mediated recurrent VF in general and in relation to the MB although multiple ablation attempts may be required.
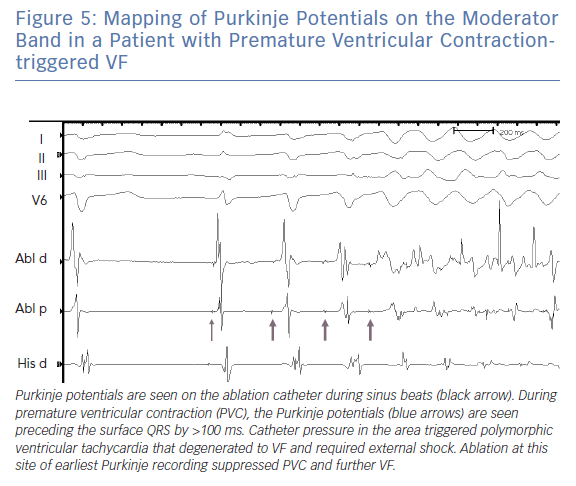
Typically, ablation for MB related arrhythmia require the use of an electro-anatomic mapping system preferably with the ability to incorporate ultrasound images (see below). In the absence of an intracardiac echocardiography, the use of a transthoracic echo to visualise the location of the MB has been reported.26 Earliest activation during PVCs is targeted and typically a Purkinje potential is evident preceding the QRS by 30–100 ms (Figure 5). Pacing at the site usually replicates QRS morphology although it is possible that varying exits between the septum and free wall can alter morphology (Figure 4). RF application may trigger repetitive PVCs or rarely, provoke VF (Figure 5).
MB ablation poses several challenges. Identification of the causative Purkinje potentials can be difficult, and effective ablation may be hindered by surrounding myocardial tissue of variable thickness and length thereby resulting in need for more aggressive ablation.13,15 In addition, as the MB is a suspended intracavitary structure, ablation is frequently limited by difficulty with catheter stability and inability to maintain consistent contact.6,10,12 Also, Purkinje-associated arrhythmias may be particularly susceptible to suppression with sedation. Nakamura et al. described the reduction of identifiable PVC targets from 92% to 63% when general anaesthesia was used as opposed to conscious sedation.9 We have used isoproterenol, epinephrine, ventricular and atrial stimulation to provoke PVCs during the procedure. Finally, the variable morphology of the MB supports different PVC-exit sites that may need to be targeted at multiple points along its length from the lateral RV wall to the anterior RV papillary muscle and interventricular septum.22,26 Interestingly, ablation at the earliest Purkinje potential on the MB usually suppresses all PVC morphologies in keeping with the concept of a common origin and varying exits.
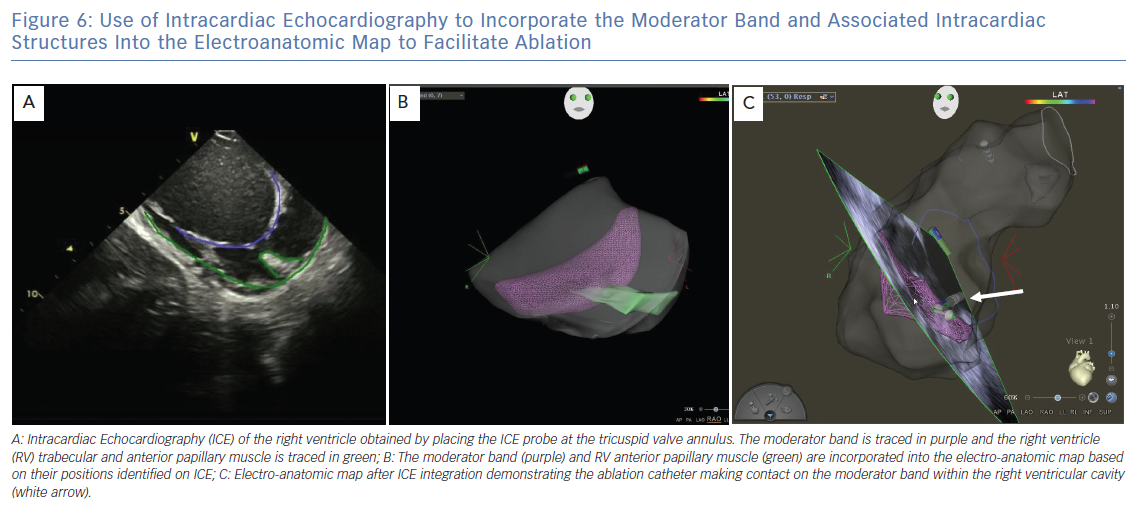
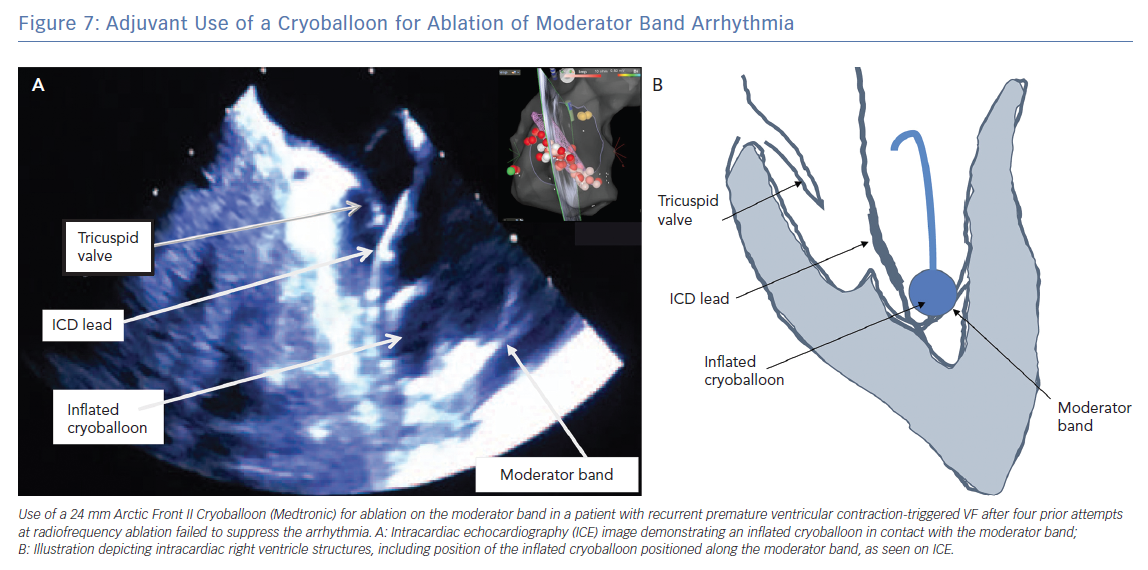
ICE is essential for visualisation and identification of intracardiac structures, such as the MB. The MB and associated papillary muscles can be identified on ICE and incorporated into the electro-anatomic map to guide and verify catheter contact along the MB (Figure 6).6 However, ICE alone may not sufficiently facilitate effective ablation in all necessary sites owing to the variable and complex structure of the MB. Effective ablation along the MB therefore may be facilitated by contact force-sensing catheters with vector analysis in addition to ICE to verify catheter position, as well as higher energy delivery to compensate for the difficulty applying steady contact. Appreciation of an impedance decrease by at least 10 ohms may be beneficial to confirm tissue heating. In one report, a cryoballoon was successfully used to facilitate consistent contact and create broad based lesions along the entire length of the MB in a patient in whom multiple prior catheter-based RF ablations had failed to suppress PVC mediated VF (Figure 7).12
Outcome in Patients with Moderator Band-associated Arrhythmias
As data on long-term results of ablation for MB arrhythmias are sparse, one has to rely on success rates for ablation of idiopathic VF as a surrogate. The long-term success rate for ablation of Purkinje mediated VF is 80% during follow up to 5 years.27 Regardless of the suppression of arrhythmias, most of these patients will need an ICD to protect against recurrent VF and sudden death as the long-term prognosis and nature of progression of these patients is unknown. Some of these patients may have associated occult endomyocardial fibrosis that facilitates the propagation of PVCs and development of fibrillation (see above). Continued follow up is essential as manifest phenotypes of known diseases may become apparent with time. Some advocate the use of genetic testing to exclude known genetic arrhythmia syndromes. However, the yield from genetic testing for a known disease specific genetic mutation in idiopathic VF is very limited, ranging from 0–9%.1
Conclusion
The RV MB is increasingly being identified as a common source for idiopathic VF. The arrhythmias that occur in association with the MB follow a course similar to other Purkinje-mediated arrhythmias. Short coupled PVCs with LBBB left superior axis morphology should alert to the possibility of the MB as a source. Mapping and ablation guided by intracardiac echocardiography can be lifesaving and highly successful in preventing recurrence. The technical difficulties of stable catheter positioning on the MB, the differential impulse exits along the septum and free wall and the importance of locating and dissociating Purkinje potential during ablation are features to be recognised for successful management of these arrhythmias.
Clinical Perspective
- The RV moderator band is a source for Purkinje-mediated arrhythmias.
- PVCs and VT from the moderator band have typical morphological features that point to it as source.
- The RV moderator band is a source of idiopathic VF triggered by PVCs that is potentially treated with catheter ablation.