Ventricular arrhythmias (VA) are commonly associated with structural heart disease and have substantial impact on patient outcomes and health system costs. Within the realm of cardiomyopathy (CM), there has been substantial progress with respect to ischaemic CM (ICM) in the understanding of infarct related scar biology and scar-mediated ventricular tachycardia (VT).
This has led to interventions to decrease VT recurrence and associated ICD shocks, including radiofrequency catheter ablation therapy.1 However, these advances have not translated into the same long-term successes for patients who present with VT in the setting of non-ischaemic CM (NICM).2 Yet, it has been increasingly recognised in VT referral centres that there is a growing number of patients referred for consideration of VT ablation secondary to NICM.3
NICM comprises a heterogeneous group of disorders and in a majority of patients the underlying cause is not identified, leading to a label of idiopathic. This is problematic for a variety of reasons since, in many instances, patients are not offered a uniform diagnostic approach and ultimately the absence of a causal entity may impair the ability to provide optimal care. Active myocardial inflammation is becoming better understood as a frequent finding in NICM and a potential contributor to poorly controlled VA.4
Definition
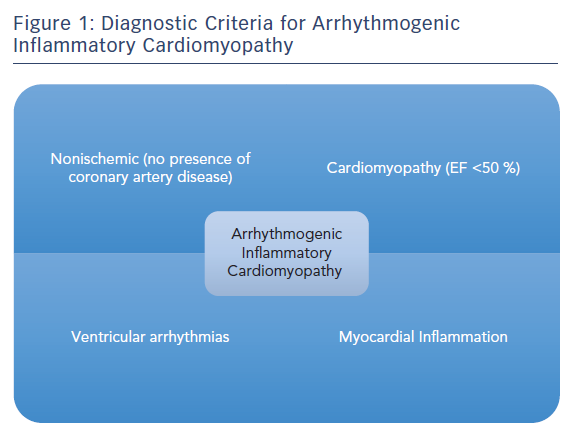
Arrhythmogenic inflammatory cardiomyopathy (AIC) is a recent clinical description encompassing a broad group of patients with NICM, who are referred to electrophysiologists for management of VA and are found to have evidence of active myocardial inflammation of unclear aetiology (Figure 1).5
Proposed diagnostic criteria for AIC are:
- Non-ischaemic cardiomyopathy with an left ventricular ejection fraction (LVEF) of <50 %.
- The presence of documented VA – monomorphic or polymorphic VT, ventricular fibrillation or frequent premature ventricular contractions.
- Patchy focal or focal on diffuse fluorodeoxyglucose (FDG) uptake on PET imaging.
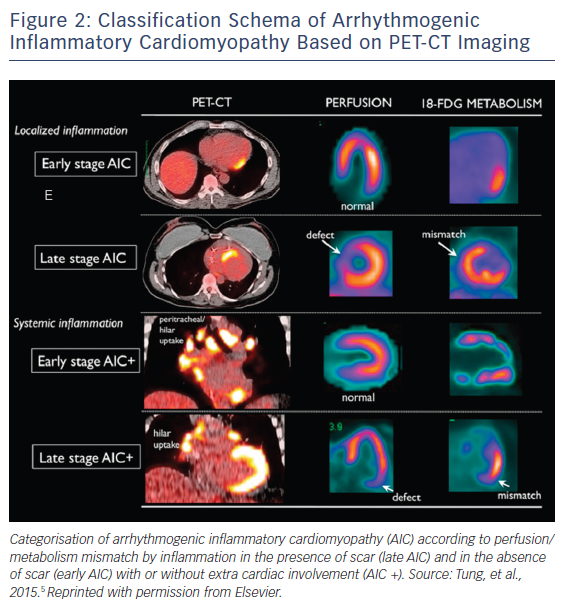
Diffuse uptake was eliminated from the diagnostic criteria, given the potential for inadequate fasting/physiologic uptake with limitation of specificity.6 AIC can be further categorised according to perfusion/metabolism mismatch by inflammation in the presence of scar (late AIC) and in the absence of scar (early AIC) with or without extra cardiac involvement (AIC+; Figure 2). AIC as a classification schema provides a useful framework for conceptualising this particular group of patients and as a reminder to the potential role that chronic inflammation plays in mediating this disease process.
Epidemiology
The prevalence of NICM is difficult to determine due to the variety of pathologies that contribute and the regional and geographic variability of those conditions. A study by Lipshultz et al. reviewed the US paediatric CM registry from 1996 to 2003 and determined that the incidence of CM was 1.13 per 100,000 children.7 In adults, one of the largest ongoing registries is the Sarcomeric Human Cardiomyopathy Registry (SHaRe) which has reported an overall prevalence of approximately 2.4 million adults living with either hypertrophic or dilated cardiomyopathy in the US and Europe. A variety of national and international registries exist for both children and adults with specific types of CM, but there is a paucity of data on the prevalence of NICM overall and more specifically on chronic myocarditis/inflammatory cardiomyopathy given the lack of large studies in these areas. The key finding from the paper by Tung et al. was that in a cohort of 103 patients with NICM and VA 49 % had underlying myocardial inflammation.5 This study is limited by referral bias given the highly sub-selected group of patients included. Nevertheless, there is likely a significant population of patients with NICM that have unrecognised chronic inflammation that requires further study.
Pathology
The pathophysiologic basis of AIC is not well understood and there has not been sufficient investigation into this newly described entity. Some patients with NICM and VA have alternative diagnoses that are made after further diagnostics are considered. An example of this would be in a patient identified as having AIC+ after evaluation of PET-CT imaging, but is reclassified as systemic sarcoidosis with cardiac involvement after intrathoracic lymph node biopsy confirms the presence of non-caseating granulomas and chronic inflammation on histology.8
In the Tung et al. study, 60 % of the patients who underwent biopsy demonstrated evidence of chronic lymphocytic infiltration and inflammation. The significance of this finding is not clear. It is likely that a role is played by an inflammatory trigger in a person with an underlying genetic predisposition towards chronic inflammation and autoimmunity, but this hypothesis will require further scientific evaluation.9
Differential Diagnoses
AIC is considered an aetiology of NICM that exists within the realm of chronic inflammatory CM. It is imperative to consider other causes of chronic inflammatory CM to ensure patients receive the appropriate diagnostic and therapeutic possibilities.
Infectious
Viral
Infectious aetiologies of chronic inflammatory CM are the most studied, with viral myocarditis being the best understood. The most common viruses associated with cardiotropism are the enteroviruses, with coxsackie B virus being the most highly implicated. A study by Donoso Mantke et al. evaluated the prevalence of cardiotropic viruses in explanted hearts of patients who underwent transplantation and found enteroviruses (mostly coxsackie B) and adenoviruses, followed by parvovirus B19, human herpes virus 6 and cytomegalovirus.10 Other viruses that cause CM include echoviruses, Epstein-Barr, hepatitis C, HIV and influenza A.
Bacterial
Bacteria are usually considered in the aetiology of endocarditis, but many bacterial species are known to cause myocarditis. Most lead to acute myocarditis that tends to recover, but some bacterial entities linger as unrecognised chronic myocarditis. Bacterial involvement tends to occur via direct inoculation, seeding from bacteraemia, or the effects of toxins produced by bacteria during the course of their lifecycle in the human host.11 Some prominent bacteria species that must be considered in the appropriate patient are Chlamydia, Corynebacterium diphtheriae, Legionella, Mycobacterium tuberculosis, Mycoplasma, Staphylococcus, Streptococcus A and S pneumoniae.
Other
Other organisms that may be considered usually depend on risk factors such as living in or travel to endemic locations, diet, age and immunosuppression status. Some of the more notable infectious agents are fungal (Aspergillus, Cryptococcus and Candida), helminthic (Trichinella spiralis) and protozoal (Toxoplasma gondii and Trypanosoma cruzi).9
Autoimmune Diseases
There are a variety of autoimmune conditions that can involve chronic myocarditis as either a primary or secondary feature and affect the heart by differing mechanisms. The various autoimmune diseases that should be considered include sarcoidosis, Churg-Strauss and eosinophilic myocarditis, giant cell myocarditis, dermatomyositis, inflammatory bowel disease, systemic lupus erythematosus, rheumatoid arthritis, scleroderma, and chronic lymphocytic myocarditis.
Drug Reactions
Drug reactions can occur by predominantly two different mechanisms: hypersensitivity/immunologic mediate and direct toxic effects. Drugs to be considered as implicated in hypersensitivity/immunologic class include penicillin, ampicillin, cephalosporins, tetracyclines, sulphonamides, benzodiazepines, clozapine, loop/thiazide diuretics, methyldopa and tricyclic antidepressants. Drugs that may have a direct toxic effect on the myocardium include amphetamines, cocaine, anthracyclines, cyclophosphamide, 5-fluorouracil, trastuzumab and phenytoin.9
Risk of Ventricular Arrhythmia/Sudden Cardiac Death and Myocardial Inflammation
Structural changes in the ventricular myocardium are implicated in the risk of VA and sudden cardiac death (SCD) in NICM. Numerous studies have implicated the degree of systolic dysfunction as having stepwise higher risks, with an EF of <35 % appropriate for primary prevention ICD implantation in most patients. Yet, in patients with AIC and other chronic inflammatory CM, data suggest that foci of inflammation and local fibrosis portend a significant risk of VA and SCD despite potentially normal or only mildly reduced systolic function.12 Mueller et al. showed that in patients with NICM with mean EF >35 %, inducibility of VA during programmed ventricular stimulation correlated significantly with areas of late gadolinium enhancement on cardiac MRI.13 A meta-analysis by Scott et al. reviewed the utility of scar quantification by cardiac MRI and identified it as a possible valuable tool in determining need for ICD therapy for primary prevention of SCD in patients with EF >35 %.14 Furthermore, the degree and localisation of inflammation as identified on PET-CT imaging has been shown to have prognostic value. Blankstein et al. demonstrated that patients with inflammation on PET-CT involving the right ventricle (RV) secondary to cardiac sarcoidosis had worse outcomes including a fivefold higher event rate with respect to malignant VA and death. This finding was postulated to be a result of a variety of possible mechanisms including RV involvement indicating greater burden of inflammatory disease and the possibility of RV inflammation being a more arrhythmogenic substrate.15 Furthermore, their study also demonstrated that areas of inflammation with concurrent perfusion defects also portend a graver prognosis. This finding mirrors the finding of Tung et al. that patients with late AIC/AIC+ had more recurrent VT despite treatment with immunosuppressive therapy and/or ablation therapy, which is likely to be related to the presence of scar.5
Diagnosis
History and Physical
The completion of a thorough history and physical examination should be the first step in assessment of patients with possible AIC. Given the nonspecific systemic manifestations of AIC, the utility of the history and physical is to identify more specific features of other disease processes (e.g. rheumatologic or autoimmune) that will guide further diagnostic testing, including laboratory and imaging.
Laboratory Testing
Laboratory tests which identify non-specific inflammation (e.g. ESR and C-reactive protein) and myocardial damage (e.g. troponin) are important both during the initial diagnostic phase and for subsequent follow up of response to therapy. Further laboratory testing should be guided based upon clinical suspicion as determined by the history and physical.
Echocardiography
Two-dimensional transthoracic echocardiography is an important diagnostic modality in AIC. While the majority of the echo findings are nonspecific, it is crucial to rule out other causes of NICM that do have more specific echo diagnostic criteria (e.g. hypertrophic cardiomyopathy). The main characteristic is systolic dysfunction with global hypokinesis. However, more specific regional wall motion abnormalities can be identified in a non-coronary artery distribution. Speckle tracking echocardiography is likely to be a useful tool as it has recently been demonstrated to identify abnormal myocardial strain patterns in early cardiac sarcoidosis, but this will require further study in AIC.16
Cardiac MRI
Cardiac MRI provides a highly specific tool for imaging inflammation and scar in patients with AIC. Cine imaging utilising steady state free precession (also known as bright blood imaging) sequences allows for evaluation of segmental wall motion abnormalities in addition to quantification of function.17 Late gadolinium enhancement (LGE) images are obtained with a pulsed inversion sequence taken 10 minutes after gadolinium contrast administration. LGE allows for identification of necrosis and replacement of viable myocardium by scar.17 Localisation patterns of LGE are important in helping to determine the potential type of AIC at play. For example, LGE in a diffuse subepicardial localisation suggests viral myocarditis, patchy basal predominant uptake suggests cardiac sarcoidosis, and endomyocardial apical predominance in eosinophilic myocarditis.18
Active inflammation can be identified utilising T2-weighted sequences to demonstrate associated oedema.19 However, there are many limitations with current T2-weighted sequences in cardiac MRI that make it less widely utilised including but not limited to: low signal to noise ratio, high dependence on magnetic field homogeneity, loss of signal due to cardiac motion in black blood preparation, motion artifact susceptibility and subjective inter-reader variability.20 Development of new magnetic resonance techniques and sequences are being investigated to minimise these limitations since cardiac MRI is the only modality to be able to noninvasively evaluate myocardial oedema, which represents early phase of myocardial inflammation. Furthermore, identification of active inflammation as compared to a preponderance of scar is likely to be important in both its prognostic value and for determination of ideal therapy (e.g. immunosuppressive therapy versus ablation), but further data are needed.
Lastly, cardiac MRI is crucial in helping to identify other causes of NICM that have a preponderance towards VA. Arrhythmogenic right ventricular dysplasia (ARVD) is commonly misdiagnosed in patients with RV involvement caused by cardiac sarcoidosis or AIC. ARVD is a predominantly hereditary condition with mutations in genes encoding for myocardial intercalated discs, most commonly desmosomal proteins.21 These abnormalities eventually lead to fibrofatty infiltration and replacement of normal myocardial tissue, predominantly in the RV, with resultant cardiomyopathy, clinical heart failure, and significant predisposition towards VA. Cardiac MRI is helpful in confirming the diagnosis of ARVD based upon the assessment of RV structure and kinetics, in addition to fibrofatty infiltration as determined utilising fat suppression sequences (e.g. fat saturation and triple inversion recovery).22
PET-CT
PET-CT imaging can be utilised with a high degree of sensitivity to identify patients with AIC.23 As previously discussed, in the Tung et al. study, nearly half of the study population were noted to have myocardial inflammation and/or perfusion defects on 18-FDG-PET-CT imaging. PET-CT cardiac imaging utilises both perfusion imaging with radiotracers 13-N NH3 or 82Rb and metabolism imaging with radiotracer 18-F FDG.24 Furthermore, CT is used for attenuation correction and improved anatomic localisation, but at the expense of increased radiation exposure with this modality. 18-FDG uptake within myocardium in the setting of no perfusion abnormality signifies a mismatch, which represents early inflammation without fibrosis. This is in contrast to 18-F FDG uptake in setting of perfusion defect or absence of 18-F FDG uptake, which represents late stage and burned out disease respectively. Recent data in cardiac sarcoidosis have demonstrated that pattern of 18-FDG uptake and perfusion defect are not only diagnostic, but can be prognostic, both in the natural course of the disease in addition to response to therapy.15, 25 This may also be true for AIC as a whole but warrants further investigation.
Endomyocardial Biopsy
Endomyocardial biopsy is an invasive approach to obtain tissue for histopathologic assessment and is still considered to be the gold standard with respect to diagnosis of myocarditis overall (infectious, autoimmune, etc). While specificity is excellent, sensitivity is poor for a variety of inflammatory CMs because of sampling error or bias.
Treatment
Currently there are no prospective or randomised controlled clinical trials specifically investigating therapies in patients with AIC. Thus, best practice for management of AIC patients is based upon literature and therapy better studied in cohorts with other aetiologies of NICM.
Beta Blockers
Beta blockers are an important class of medications in the management of chronic heart failure with reduced ejection fraction (HFrEF). Furthermore, it has been found in a small number of studies that the specific agent chosen may play an important role. In a rat model with inflammatory CM, carvedilol was demonstrated to be cardioprotective due to suppression of inflammatory cytokines whereas both metoprolol and propranolol were not.26 Beta blockers have also been noted to have an independent survival advantage in patients with VA who don’t already have an ICD, which is likely to be a large proportion of AIC patients.27
ACE Inhibitors and ARBs
Angiotensin-converting enzyme (ACE) inhibitor and angiotensin-receptor blocker (ARB) therapy are well established in HFrEF management, with early initiation helpful in minimising maladaptive ventricular remodelling and have demonstrated substantial survival advantage. There are small mouse model studies that have demonstrated this in autoimmune myocarditis for ACE inhibitors and ARBs, but further studies are warranted specifically in patients with AIC.28,29
Aldosterone Antagonists
Aldosterone antagonists have an increasing role in the management of HFrEF patients both for ischemic and non-ischaemic aetiologies of disease. There are small mouse model studies that demonstrate anti-inflammatory benefits in viral myocarditis, but there have not been studies in our review of these agents in virus negative chronic myocarditis or for AIC patients in particular.
Immunosuppressive Therapy
Immunosuppressive therapy has been a controversial topic in the management of inflammatory CM overall. There have been a variety of trials using heterogeneous methods, study populations, and outcome measures that have provided little further clarification. In 1989, Parrillo et al. published one of the earliest trials evaluating prednisone in idiopathic dilated CM.30 This was a randomised controlled trial of 102 patients with idiopathic dilated CM and endomyocardial biopsy demonstrating inflammatory features on histopathology. Patients were assigned to prednisone or placebo, with the prednisone arm demonstrating a statistically significant 2.2 % absolute improvement in LVEF. The improvement in LVEF was the primary outcome measurement, as the study was not powered to evaluate survival benefit.
Subsequently, the 1995 European Study of Epidemiology and Treatment of Cardiac Inflammatory Disease (ESETCID) examined 182 acute or chronic myocarditis patients with LVEF <45 %.31 Patients with a cytomegalovirus, enterovirus or adenovirus were given antiviral or immunoglobulin versus placebo. Subjects with virus negative myocarditis were administered prednisolone and azathioprine or placebo. The primary outcome was reduction in inflammation, but no statistically significant benefit was seen in either the virus positive or virus negative arms versus placebo .
The Myocarditis Treatment Trial (MTT), published in the same year as the ESETCID trial, was a multicentre randomised controlled trial of 111 patients with myocarditis and LVEF <45 % assigned to conventional heart failure therapy or immunosuppressive therapy with prednisone plus cyclosporine or azathioprine.32 This study was designed to evaluate mortality benefit, but there was no benefit demonstrated at the conclusion of the trial. In 2009, the Tailored Immosuppression in Inflammatory Cardiomyopathy (TIMIC) study was published, which was a randomised controlled trial of 85 patients with biopsy proven virus negative inflammatory CM assigned to prednisone and azathioprine or placebo over a 6-month period.33 The immunosuppression arm of this trial did demonstrate a statistically significant improvement in LVEF and reduction in LV chamber dimensions as compared to the placebo arm.
In the Tung et al. study of AIC patients, it was retrospectively discovered that subjects with early AIC and AIC+ demonstrated greater benefit with respect to reduction in recurrent VT with the use of immunosuppressive therapy compared to late AIC over a 6-month period.5 This may be explained by immunosuppressive therapy having greater benefit in the setting of active inflammation before the development of scar later in the disease course.
There are significant limitations with past studies due to heterogeneity of included patients with acute, subacute, and chronic myocarditis; issues with interpretation of endomyocardial biopsy results within certain studies; and choice of immunosuppressive therapy. Further studies are warranted to tease out specific groups of patients including AIC patients that may in fact benefit from early and/or long-term immunosuppressive therapy.
Despite these limitations, a trial of immunosuppressive therapy could be considered in patients with suspected AIC as determined by clinically proposed criteria and supported by advanced imaging (PET-CT and/or cardiac MRI). Based upon the work of Tung et al., patients presenting with VT storm can be induced with two doses of methylprednisolone 1g IV, followed by 40 mg oral prednisone daily. Patients without VT storm can be started on daily prednisone without pulsed IV dose. After 8 weeks, repeat PET-CT imaging to assess for response in addition to clinical assessment of reduction in arrhythmia burden. If improvement noted, then consideration for tapering dose of prednisone by 10 mg every 1−2 months with gradual transition to a steroid sparing immunosuppressant agent (e.g. azathioprine, etc.).5
Implantable cardioverter-defibrillator therapy
ICD therapy has been a cornerstone of HFrEF management since pivotal trials such as the Multicenter Automatic Defibrillator Implantation Trials (MADIT and MADIT-II), and Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) had demonstrated reduced mortality over 1−5 years follow-up.34−36 Those studies were a composite of both ICM and NICM and thus difficult to ascertain the benefit for NICM and more specifically AIC. Most recently, the Danish Study to Assess the Efficacy of ICDs in Patients with Non-ischemic Systolic Heart Failure on Mortality (DANISH) study of 556 patients with NICM and symptomatic heart failure found that there was an insignificant reduction in the primary outcome of all-cause mortality, but with a statistically significant reduction in SCD with ICD over control.37 While this trial provided more specific data as to the benefit of ICD therapy in patients with NICM, a major limitation with this study and a reflection of the field in general, is the lack of stratification of types of NICM.
The field cannot be oversimplified into ICM versus NICM without missing the mark on how best to manage patients with specific forms of NICM. For instance, hypertrophic CM and AIC are both considered specific types of NICM that can manifest with malignant VA. In the field of hypertrophic CM, there are fairly strict evidence-based guidelines as to the use of ICD therapy. However, these data and guidelines cannot be used in patients with AIC given the significant differences in their pathophysiology and clinical manifestations. Even within the field of inflammatory cardiomyopathies there exists significant differences between specific causes based upon the natural course of the disease process that makes risk stratification of sudden cardiac death difficult.
Giant cell myocarditis is a very rare form of inflammatory CM that is usually fatal within 6 months without heart transplantation. Therefore, while ICD therapy in these cases may avert SCD, it would not be expected to change the long-term expectation and survivability of the underlying disease. In addition, other diseases such as cardiac sarcoidosis and likely AIC, can have relapsing/remitting courses and the natural progression may be affected by immunosuppressive therapy. Thus, it is not known how ICD therapy would benefit these individuals with respect to improving long-term survival. There is a significant paucity of data in the field of ICD therapy in subgroups of NICM and at this time clinicians must use currently published guidelines and their best clinical judgement to have a discussion with their patients about using this particular therapy.
Ablation Therapy
The role of radiofrequency ablation in patients with AIC is not well studied or understood. It has been reported in the literature that there is a clear discordance between long-term ablation outcomes in ICM and NICM. This difference is thought to be due to a lack of modifiable substrate (scar) in NICM patients. NICM patients may have a combination of scar-based re-entrant VT and functional VT not directly related to myocardial scar or fibrosis. Kumar et al. investigated the characterisation of substrate and outcomes after ablation in 435 patients with cardiac sarcoidosis. They identified that the mechanism of cardiac sarcoid-related VT is likely to be a result of re-entry involving confluent regions of scarring in the RV endocardium and epicardium along with patchy LV endocardial scarring affecting the basal septum, anterior wall and perivalvular regions. Furthermore, catheter ablation was able to terminate VT storm and >1 inducible VT in the majority of patients, resulting in reduction in ICD shock burden, but recurrences were common and failure to abolish all VT was predominantly attributable to intramural circuits.38 Patients with AIC may be prone to VA related to scar, but possibly also VA directly caused by inflammation. Since patients with AIC can develop VA across the spectrum of early to late disease, it will be of great importance to further study the outcomes of these patients to best determine the safety and efficacy of an ablation versus ablation plus immunosuppressive strategy for management during various stages of the disease process.
Conclusion
AIC is a recent clinical description encompassing a broad group of patients with NICM, who are referred to electrophysiologists for management of VAs.5 These are a unique group of patients who suffer from long standing chronic myocardial inflammation and myocardial scar formation that leads to congestive heart failure and the development of malignant ventricular arrhythmias. Currently, much of the literature to help diagnose and manage these patients is extrapolated from patients with NICM and sarcoidosis, so it is of paramount importance that further effort is made to investigate patients with AIC and to establish optimal diagnostic and treatment paradigms.
Clinical Perspective
- A significant number of patients with non-ischaemic cardiomyopathy and ventricular arrhythmias have been referred to electrophysiologists with underlying myocardial inflammation of unclear aetiology.
- Arrhythmogenic inflammatory cardiomyopathy is a newly described entity encompassing this group of patients to catalyse a paradigm shift in clinical care and promote the need for further research in the field.