AF is the most common sustained cardiac arrhythmia in both healthy individuals and patients with structural heart diseases. AF is a concern due to its association with increased morbidity and mortality, as demonstrated in large epidemiological studies, and the incidence of AF increases generally with age.1–4 AF can occur as a complication in acute MI (AMI), with incidences ranging from 6% to 21%.2 Previous AF may also increase the risk of MI itself.5 Arrhythmic or high-frequency ventricular contractions during AF can worsen coronary blood flow and ventricular function. However, most of the time, the arrhythmia is well-tolerated in MI patients, and besides anticoagulation, no specific treatment is necessary according to the 2023 European Society of Cardiology guidelines for acute coronary syndrome.2,6 Nevertheless, AF in the context of AMI can sometimes lead to complications, such as cardiogenic shock, ischaemic stroke and secondary MI, resulting in an increased mortality rate.1,7
Some prior studies did not find an independent impact on the prognosis of patients with MI complicated by AF, while other studies did, making the effect on patient outcomes a controversial topic.8–12 Importantly, the majority of prior data are obtained from clinical studies in selected hospitals, and the included cases may not accurately represent the cases diagnosed in a defined population.13 Additionally, most studies focused on ST-segment elevation MI (STEMI) patients, with only a few authors including non-STEMI (NSTEMI) cases in their analysis. Therefore, the purpose of this study was to investigate the impact of AF in the admission ECG on long-term all-cause mortality in patients with incident AMI based on data of a population-based MI registry.
Material and Methods
Study Sample
The present study used data from the population-based Myocardial Infarction Registry Augsburg, situated in southern Germany. This registry has been systematically compiling data since 1984, initially as part of the Monica project (Monitoring Trends and Determinants in cardiovascular Disease). Following the conclusions of the Monica project in 1995, the registry continued its operations under the nomenclature of the KORA Myocardial Infarction Registry; from 2021, it functioned under the designation of the Myocardial Infarction Registry Augsburg. Geographically, the study area encompasses the city of Augsburg and two adjacent counties, representing a total population of approximately 680,000 individuals.
For this investigation, all cases of hospitalised incident AMI occurring between 2009 and 2017 within the study area were included in the analysis. Suitable patients were required to have their primary residency within the designated study area and fall within the age range of 25–84 years. Only individuals surviving beyond the initial 28 days after hospital admission were included in the present analysis to focus on long-term all-cause and cardiovascular disease (CVD) mortality.
Patient data were meticulously collected by trained study nurses using standardised questionnaires administered during the patient’s hospitalisation period. In addition, medical chart reviews were undertaken to supplement the data set with clinically relevant information. A comprehensive dataset was created for each case of incident AMI, encompassing the type of MI, laboratory values on infarction size and kidney function, cardiovascular risk profiles, comorbidities, therapeutic interventions, sociodemographic attributes, and pharmacological therapy at hospital discharge. For a large proportion of the admission ECGs, whether AF or sinus rhythm (SR) was present was recorded. Detailed documentation of the methodologies used for data collection and variable definitions within the Myocardial Infarction Registry Augsburg can be found in prior publications.14–18
Inclusion and Exclusion of Cases: Sample Size
Only patients with incident AMI were included (STEMI, NSTEMI, new left bundle-branch-block). MI was defined as acute myocardial injury through the detection of dynamically elevated cardiac biomarkers concurrent with evidence indicative of acute myocardial ischaemia.19 Only patients with rhythm data on the admission ECG were included. Cases were excluded if they showed incomplete data regarding the following variables: sex, age, BMI, duration of follow-up, left ventricular ejection fraction, arterial hypertension, smoking status, diabetes, hyperlipidaemia, serum creatinine levels, percutaneous coronary intervention (PCI), history of bypass surgery, presence of typical chest pain, and receipt of antiplatelet agents, statins, angiotensin-converting enzyme inhibitors, angiotensin II inhibitors or β-blockers as part of MI-specific pharmacological therapy at discharge. After the application of these exclusion criteria, a total of 2,313 patients were included in the statistical analysis. Baseline characteristics of the study sample can be found in Table 1.
Out of the 2,313 included patients, 2,157 patients showed regular SR, and 156 patients showed AF, which was defined as a supraventricular arrhythmia characterised by uncoordinated atrial electrical activation, followed by ineffective atrial contraction. ECG manifestations of AF typically contained irregular R-R intervals, provided that atrioventricular conduction remained unimpaired. Additionally, the absence of repeating P waves and irregular atrial activation pattern are ECG features of this arrhythmia.5 Whereas atrial flutter is a common arrhythmia that usually accompanies AF itself, only a small percentage of patients have isolated atrial flutter.8 Therefore, patients with atrial flutter were considered to have AF in the underlying study.
BMI classifications included normal weight (BMI ≤24.9 kg/m²), overweight (BMI 25−29.9 kg/m²) and obese (BMI ≥30 kg/m²). Renal function was stratified based on serum creatinine levels as follows: normal (0–1.00 mg/dl), slightly impaired (1.01–1.50 mg/dl), moderately impaired (1.51–2.00 mg/dl) and severely impaired (≥2.01 mg/dl). The type of AMI was characterised by physicians based on the admission ECG. Infarct size was estimated using the peak creatine kinase myocardial band (CK-MB) level (U/l) during hospitalisation. Infarction size was categorised as small (CK-MB: 0–150 U/l), intermediate (151–300 U/l), large (301–600 U/l) and extensively large (≥601 U/l). Of note, 289 patients with unknown CK-MB levels were kept within the study population as a distinct subgroup. Estimated GRACE score was calculated using the web calculator from Walker et al.20 The calculation was based on a formula provided by Andersson and FitzGerald.21 The exact formula can be found on their website at sub-point 8: “Risk score for FOX prediction of death or MI from admission to 6 months later”.21
Outcome
The primary endpoint of the cohort was long-term all-cause mortality after the initial primary MI. Further endpoints were CVD and CHD mortality. Longitudinal tracking of survival status was maintained through regular follow-up assessments. Therefore, death certificates were procured from local health and registration authorities, with the most recent mortality follow-up for this study made in 2019. Death certificates were coded for the underlying cause of death by a single trained person using the ICD-10. The causes of death were grouped as follows: all-cause mortality (A00-U85), CVD mortality (I00-I99) and ischaemic heart disease mortality (I21-I25).
Statistical Analysis
Categorical variables were calculated as totals and percentages. Means and SDs were used for continuous variables, and the median with interquartile range (IQR) for serum creatinine and peak CK-MB level. Statistical comparisons between the SR and the AF groups were conducted using χ2 tests for categorical variables. Student’s t-tests were employed for continuous variables: age, BMI, follow-up time and GRACE-score, while the Mann–Whitney U-test was used for peak CK-MB and serum creatinine levels.
To calculate the impact of AF on long-term mortality, different Cox regression models were performed. The primary model assessed the effect on long-term survival, using only the categorical variable admission ECG rhythm without any further adjustments. The secondary model included adjustments for sex and age, while the tertiary model further incorporated the additional variables of diabetes, arterial hypertension, BMI, smoking status, hyperlipidaemia, type of MI, localisation of MI, left ventricular ejection fraction (LVEF) ≤30 versus >30%, typical chest pain on admission, recanalisation by PCI, bypass surgery or peak CK-MB and serum creatinine levels. Also, medication at hospital discharge was referred to, depending on how many of the four evidence-based substances for AMI were prescribed, comprising angiotensin-converting enzyme inhibitors/angiotensin II blockers, antiplatelet agents, statins and β-blockers.17 The proportional hazards assumptions were assessed visually through log-minus-log transformed survival plots, indicating no significant violations for any of the variables used.
Statistical analyses were conducted using SPSS version 29.0.1.0 (IBM), with significance set at p<0.05. We performed the same three Cox regression models, with CVD and coronary death as specific endpoints (cause-specific Cox regression models).
Results
The baseline characteristics of patients with incident AMI stratified by ECG rhythm are detailed in Table 1. Most patients were men (71.2%). Notably, AF patients were older (mean age 73.6 versus 64.1 years) and included a higher proportion of women (35.3 versus 28.3%) compared with the SR group. Baseline characteristics showed a significantly higher prevalence of arterial hypertension (90.4 versus 75.3%; p<0.001) and diabetes (38.2 versus 29.8%; p=0.023) in AF patients, while current smoking was less common in this group (12.8 versus 35.1%; p<0.001). AF patients presented more frequently with NSTEMI (62.8 versus 52.5%; p=0.016), whereas SR patients were more likely to have STEMI (41.9 versus 30.1%; p=0.016).
Analysis of infarction characteristics revealed that initial AMIs in AF patients were significantly smaller than those in SR patients, indicated by peak CK-MB levels (median serum level 54.0 versus 76.0 U/l in the SR group; p=0.005), possibly due to a higher incidence of transmural STEMIs in the SR cohort. The estimated GRACE score was significantly higher for AMI patients with AF (112.8 versus 138.5; p<0.001). Additionally, AF patients more frequently revealed an impaired kidney function, as indicated by elevated median serum creatinine levels (1.11 versus 0.98 mg/dl). Regarding the treatment, the majority in both groups underwent PCI, but AF patients were less likely to undergo PCI than SR patients (73.7 versus 80.0%), a difference that did not reach statistical significance (p=0.059).
At hospital discharge, most patients were prescribed a combination of four evidence-based medications. A total of 59.0 % of AF patients received oral anticoagulation (OAC) in their discharge medication. For SR patients, just 10.6% of cases were prescribed an OAC. For the 132 patients with LVEF <30%, we showed that a total of seven (5.3%) individuals received an ICD and/or a CRT device. From the 2,180 patients with LVEF >30%, a total of nine (0.4%) patients received an ICD and/or a CRT device (Supplementary Table 1). For one patient with LVEF >30%, no data about ICD/CRT implantation were available.
Cox Regression Analysis
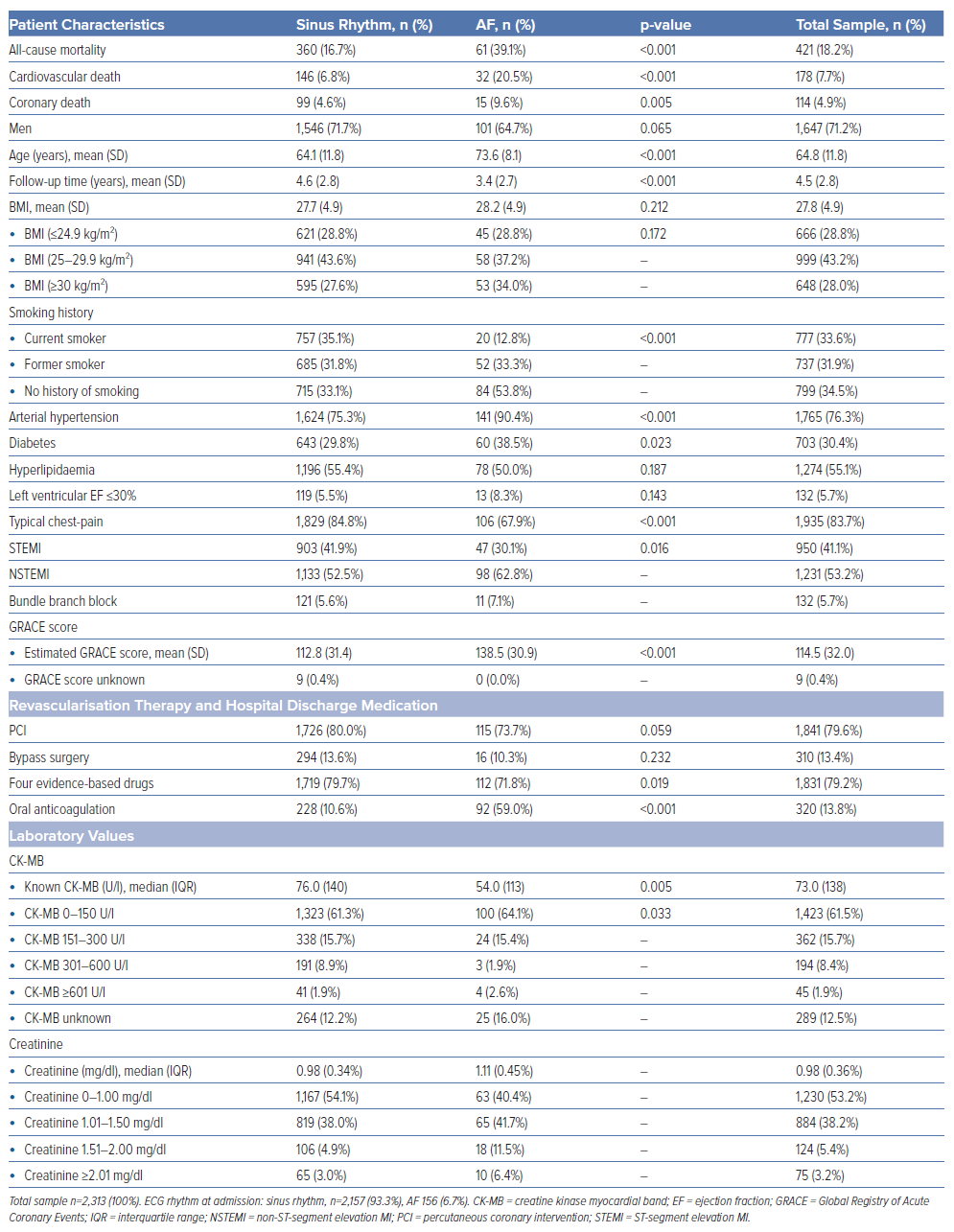
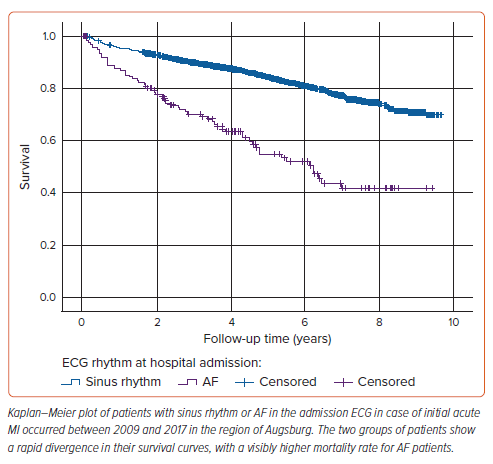
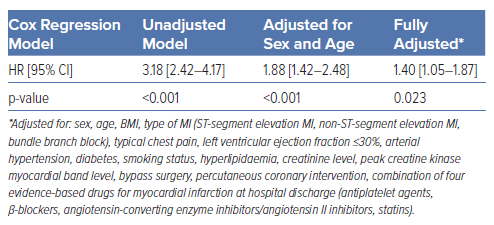
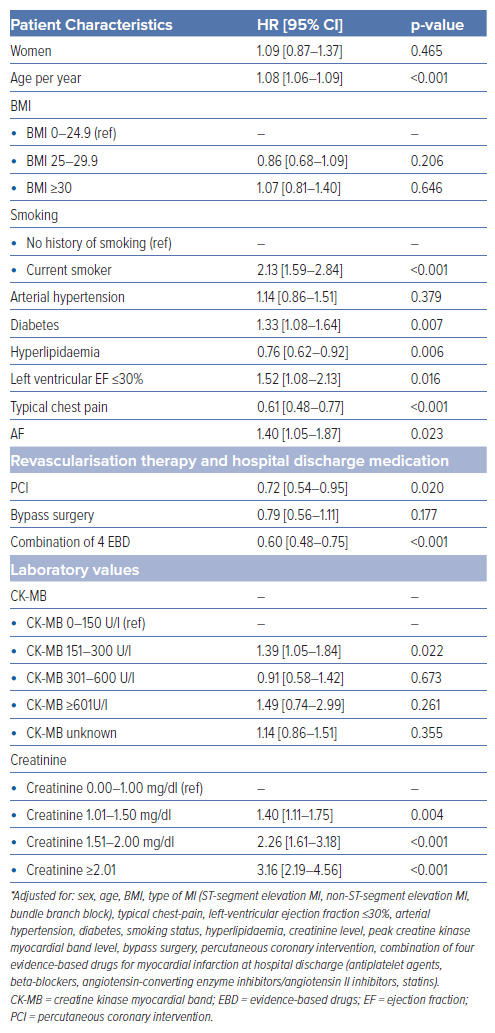
Survival analysis revealed a significantly higher long-term mortality rate in AF patients (39.1%) compared with those with SR at admission (16.7%). The survival curves for both groups are shown in Figure 1. The survival disparity persisted across different Cox regression models (Table 2), but it was clearly attenuated after adjusting for other relevant variables. In the unadjusted Cox regression model, we yielded a HR of 3.18 (95% CI [2.42–4.17]), while adjustment for age and sex resulted in a HR of 1.88 (95% CI [1.42–2.48]). In the fully adjusted model, AF patients still had a significantly higher long-term all-cause mortality with a HR of 1.40 (95% CI [1.05–1.87]). The HRs and p-values of other relevant covariables included in the fully adjusted model are displayed in Table 3. A total of 178 patients died due to CVDs, 114 patients showed a coronary-based death. For AF patients, the analysis with CVD death (Supplementary Tables 2 and 3) as the outcome in the fully adjusted model showed a HR of 1.68 (95% CI [1.11–2.55]; p=0.015). For coronary death as the specific endpoint, no significant increase in mortality for AF patients was found; the HR was yielded here at 1.16 (95% CI [0.64–2.09]; p=0.622; Supplementary Tables 4 and 5).
Discussion
The present study showed that the presence of AF on the admission ECG was associated with a worse long-term survival after an acute primary MI in hospitalised patients aged 25–84 years even after adjustment for a variety of confounders. Further analysis showed a significantly higher CVD mortality for AF patients, but could not find an independently increased risk of coronary deaths, even though AF might generally lead to a worsening of coronary blood flow due to irregular ventricular contractions.2 We interpreted the significantly increased risk of CVD death probably due to higher rates of congestive heart failure and thrombo-embolic events.
Several prior studies reported associations between previous or new-onset AF and worse long-term outcomes in cases of AMI from the pre-thrombolytic, the thrombolytic and the PCI era.1,8,13,22–31 Most of the cited studies found a significantly higher average age for AF patients compared with SR patients, as we did in our study.1,8–13,22–31 In our sample, patients with AF were on average 9.4 years older than those with SR. Previous studies have reported a higher burden of cardiovascular risk factors and comorbidities in the AF group.1,10,11,13,22,26–31 Renal impairment has also been associated with AF in cases of AMI;1,13,22 our findings supported these reported associations. The AF group in our sample exhibited a higher proportion of women, a phenomenon previously observed by other authors.1,11–13,26–31
No significant difference was found between elevated BMI and AF in the admission ECG in our study. Pederson et al. also did not show any differences in BMI between the SR and AF group.27 However, Vukmirović et al. found significant associations between elevated BMI and AF in AMI cases.8
In our sample, the manifestation of NSTEMI showed a significantly higher proportion in the AF group. This could be explained by the fact that NSTEMI patients, on average, were older (73.6 years), compared with the relatively young STEMI patients (64.1 years) in our study. Other authors also found higher NSTEMI rates in patients with AF.1,23 Few authors included atrial flutter as a complication of AMI.9,13,27–29 Labre et al. evaluated atrial flutter in AMI similarly to AF, as we did in our analysis.13
Some studies reported higher rates of left ventricular impairment in AMI patients with AF.10,11,30,31 Accordingly, we found higher rates of patients with LVEF <30% within the AF group, but without reaching statistical significance.
We observed that patients with LVEF <30% received an ICD and/or CRT device after AMI significantly more often during the acute hospital stay. The implantation rate of 5.3% seems to be very low for these patients during the initial hospitalisation. The overall implantation rate might be much higher due to an unknown amount of elective ICD and/or CRT implantation within a second hospital stay.
Most of the AF patients (59.0%) received OAC at hospital discharge in our analysis; this rate was almost sixfold higher than the OAC rate of SR patients at discharge (10.6%). It must be noted that still 41.0% of the AF patients did not receive OAC. This high rate might indicate that many AF patients were not adequately protected against thrombo-embolic events at hospital discharge. A few patients probably did not receive OAC due to general bleeding risk, but it is questionable if this is true for all 41.0% of AMI cases.
The baseline characteristics indicated that patients with SR are threefold more likely to be active smokers than those with AF. This finding is consistent with the results of Wong et al. and Crenshaw et al.11,29
Few authors could not show worse long-term outcomes in AF patients. For example, Kinjo et al. did not find significantly worse 1-year survival for AMI patients with AF at hospital admission.9 Also, Asanin et al. did not observe significantly worse 7-year survival for patients with early-onset AF in the setting of AMI (AF onset <24 hours after the beginning of symptoms) compared with patients with SR and AMI. Interestingly, Asanin et al. and Crenshaw, et al. reported significantly larger infarct sizes within their AF groups, estimated by peak creatine kinase levels. In contrast, we found significantly smaller infarct sizes in AF patients according to peak CK-MB levels, likely due to a smaller amount of transmural infarction in the AF group when compared with those with SR.10,11 Crenshaw et al. did not observe a significantly worse 1-year survival in patients with AF at hospital admission after adjustment for relevant covariables.11 Similarly, Goldberg et al. in 1990 examined a population-based study sample of 4,108 AMI patients, but were not able to detect an independently worse long-term survival for patients who developed AF during hospitalisation. Of note, they excluded patients who developed AF in the first 24 hours of hospitalisation, so their results are not fully comparable with ours, as we were referring to AF on admission ECG.12
Most of the studies reporting no worse long-term outcome in AF patients were from the early 2000s or 1990s, or even older. The more recent studies, in times when PCI was widely available, mostly showed a worse long-term outcome in AF patients. This raises the question of whether AF complicating AMI is becoming a more serious risk factor for death in an era of better AMI prognosis itself, due to coronary angioplasty and evidence-based pharmacological treatment options.32
However, there are also contemporary studies that can be compared with our results. For example, in a nationwide French analysis from 2020, including 797,212 AMI patients, MI occurred between 2010 and 2019. At a median follow-up time of 0.7 years (interquartile range 0.1–3.1 years), 9.5% of the patients showed previous AF, and new-onset AF was diagnosed in 4.4% of the patient sample. Significantly higher mortality rates for patients with AF were found.23 In general, the prognosis for new-onset AF was worse than for patients with previous AF (adjusted HRs 1.174 and 2.110, respectively). These results seem comparable to our HR of 1.40 in the fully adjusted model, but we were not able to differentiate between new onset and previous AF, which might have influenced the results.23
In a recent study from 2017 by Vukmirovic et al., 600 patients with AMI were examined, of whom 48 developed AF during their hospital stay. At the 84-month follow-up, the mortality rate was 64.6% for AF patients and 39.1% for those without AF. A non-significantly higher mortality rate was observed in the AF group. That study did not include patients with pre-existing AF before hospitalisation, making its results difficult to compare with ours.8
Another contemporary study conducted by Podolecki et al. investigated 4,099 recanalised STEMI cases (median follow-up 69.7 months), excluding pre-existing AF patients, but including those with new-onset AF. They found a worse long-term prognosis for patients with anterior STEMI and new-onset AF compared with STEMI patients without this rhythmic disorder. For nonanterior STEMI, only patients with late-onset AF had worse long-term survival. The authors explained their findings by the negative impact of AF on coronary perfusion, cardiac haemodynamics and a thrombogenic effect of AF.22 This study design differed to ours, mainly due to the exclusion of previous AF patients, making their results only partially comparable to ours.
In 2009, Lau et al. investigated 3,393 patients with acute coronary syndrome, and found that patients with new-onset or pre-existing AF were significantly older. Patients with previous AF had significantly worse long-term survival with a HR of 1.42. Cases with new-onset AF only showed a significantly worse composite outcome, including stroke, MI and death.24 This study included previous AF in contrast to Podolecki et al., in addition, their HR of patients with previous AF was similar to our HR in the fully adjusted model of 1.40.24
In a recent study by Zotto et al., 1,455 STEMI patients receiving PCI, of whom 102 patients developed new-onset AF, were examined.25 The study found that AF patients had significantly worse long-term survival at a median follow-up time of 1,820 days. Additionally, the AF group was significantly older than the group without AF.25 It is to be mentioned, that their study design differed by the focus on STEMI patients, making their results partially comparable to our study.
It is important to note that AF patients are in general further characterised by different types of AF, such as paroxysmal, persistent and permanent AF. Since permanent ECG monitoring is available for certain patients carrying cardiac implantable electronic devices, the concept of AF burden arises. AF burden means the average amount of time a patient spends in AF rhythm during a certain investigation period. Botto et al. showed in their analysis that patients with a high AF burden are at especially high risk of mortality, when compared with those having a small AF burden.33 It is to be mentioned that patients carrying a cardiac implantable electronic device usually have previously existing cardiac diseases.33
Another new parameter for specific risk stratification of AF patients is the concept of post-extra systolic potentiation (PESP) transferred to irregular QRS intervals with consecutive undulation of pulse wave intensity. PESP is sometimes detectable in patients with congestive heart failure, and occurs due to pathological changes in the calcium metabolism of the myocardium. As a study from Steger et al. from 2016 demonstrated, congestive heart failure patients are at higher risk of mortality, if they show PESP.34
In a small subsample of 36 AF patients with previous AMI, they tried to project this phenomenon on AF patients with irregular heartbeat intervals and called it PESPafib. The different pulse wave intensities were measured by changes in blood pressure. They defined PESPafib if a pulse wave interval of <80% of the baseline interval duration was followed by an interval of >140%, with a potentiated pulse wave of the latter one. If the patients showed PESPafib, they had a significantly higher risk of mortality compared with those AF patients showing now potentiation of pulse wave intensity.34
In our study, we had no further information to characterise AF type, burden or PESPafib during AF. It could be an interesting aim for further investigations to include these variables in a comparable sample of AMI/AF patients to identify individuals of especially high risk, and in consequence, to treat these patients aggressively.
Contrary to prior comparable studies, this study used population-based data including all consecutive AMI patients admitted to hospital from a defined study region, which reduces the chance of referral or selection biases.35,36 Moreover, the analysis was characterised by a long follow-up time (median 4.5 years). We also had detailed information on multiple relevant covariables that were assessed in a standardised manner, and that allowed us to calculate multivariable adjusted Cox regression models. In addition to all-cause mortality, we had data on cause-specific mortality, so that we were able to conduct further analyses to investigate the association between AF and CVD, as well as CHD mortality.
Another special feature of our study was that the mortality outcomes were recorded by carrying out regular mortality follow-ups via the residents’ registration offices and making the death certificates available from the responsible health authorities. This ensures not only the complete collection of endpoints, but also very valid information about the cause of death.
Nevertheless, there are some limitations to mention. We had no information on the ratio of AF/flutter within the patient sample, we also did not know how many patients developed new-onset AF and how many patients had previous AF. Because we only regarded the admission ECG rhythm, we had no information about how many patients developed AF during hospital stay or follow-up. In addition, the information of treatment with OAC was available at discharge from hospital. It is unclear to what extent OAC medication was initiated during follow-up or whether the discharge medication was continued. No data on bleeding events, repeated PCI and heart failure hospitalisations was available in the present study. Furthermore, our findings may not be generalised to all ethnic groups or patients aged older than 85 years. Finally, we might not have considered all relevant confounders and no conclusions about causality are possible.
Conclusion
AF was significantly associated with a higher burden of other cardiovascular risk factors and comorbidities, such as hypertension, diabetes and impairment of renal function. We found an independent and significant impact on worse long-term survival of AF rhythm in the admission ECG when patients had their initial AMI. Therefore, it seems to be necessary to treat AF after AMI aggressively to reduce early mortality. For physicians, it seems important that they consider during hospital stay which patients need anticoagulation, which patients are possible candidates for rhythm control by electrocardioversion or pulmonary vein isolation and which patients need a more advanced pharmacological heart-rate control.