AF is the most common cardiac arrhythmia with a complex and multifactorial pathophysiological background. In their seminal publication, Haissaguerre et al. shed light on the role of rapidly firing pulmonary vein sources in the initiation of AF paroxysms.1 Apart from the role of pulmonary vein-triggering foci, underlying electrical and structural remodelling also contributes to the onset and perpetuation of AF, especially in advanced stages of its natural course. The term ‘electrical remodelling’ mainly reflects the modification of the electrophysiological characteristics of atrial myocytes, while the hallmark of ‘structural remodelling’ is the underlying atrial fibrosis. Interestingly, these two components of atrial remodelling exhibit a substantial mechanistic interplay. The fundamental role of fibrosis in the pathogenesis of AF has attracted intense research interest with evident clinical implications for pertinent management strategies.
Fibrosis is characterised by the proliferation of fibroblasts, which subsequently differentiate into myofibroblasts secreting extracellular matrix proteins (collagen). The disorganised myocardial architecture and cellular content promotes arrhythmogenesis in multiple ways.2 Fibroblasts and myofibroblasts may couple electrically to neighbouring cardiomyocytes, modifying their electrical properties and promoting automaticity and ectopic firing.3 In addition, the expanded extracellular matrix disrupts normal electrical cellular coupling, thus enhancing microstructural discontinuities and intensifying directionally dependent variation of conduction velocity. The latter augmentation of tissue anisotropy increases the susceptibility to unidirectional block and re-entry, and contributes to triggering and maintenance of AF.4,5 Experimental data further supported the key role of atrial fibrosis, demonstrating that regions of fibrosis or scar may anchor fibrillatory rotors,6 while re-entrant drivers in persistent AF are confined to specific regions that constitute boundary zones between fibrotic and non-fibrotic tissue.7
Preprocedural Identification of Atrial Fibrotic Areas
A method that has been used in limited centres for the identification, localisation and quantification of atrial fibrosis is late gadolinium enhancement (LGE) MRI. The contrast agent accumulates in the extracellular space owing to altered washout kinetics compared with normal tissue, resulting in a higher signal intensity in fibrotic areas due to abundant extracellular space.8 Specific MRI protocols have been introduced and clinically tested for imaging of atrial fibrosis.9,10 The identified fibrotic areas are colour coded on the surface of the left atrial shell, allowing the quantification and subsequent staging of left atrial fibrosis based on the ratio of the volume of the fibrotic left atrial wall (delayed enhancement) to the total left atrial wall volume.9,11 The value of MRI for non-invasive assessment of underlying atrial fibrosis is also supported by data showing that histological findings of collagen content in surgical biopsy specimens correlate with respective tissue characterisation by LGE-MRI.10 Several studies have also shown a high level of agreement between regions of scar identified by LGE-MRI and low-voltage areas identified by electroanatomic voltage mapping.12–14 However, contradictory results have also been reported. In cohorts of patients subjected to AF ablation, the highest LGE coverage is located at the left pulmonary vein antral, left lateral and left posterior wall,15 while low-voltage zones are preferentially distributed in the anterior wall, septum and posterior wall.16 Interestingly, the reported correlation between different techniques used for fibrosis delineation is influenced by the previous history of catheter ablation, since ablated atrial tissue is more easily identifiable by MRI, contrary to the non-iatrogenic diffuse interstitial atrial fibrosis.17 Despite recent progress in the reproducibility of left atrial fibrosis assessment,18 inherent caveats mainly stem from the reduced thickness of the atrial wall compared with the spatial resolution of the MRI. Additional limitations that may impair image quality and accuracy in LGE quantification include subjectivity in the definition of left atrial borders during segmentation, irregular rhythm and respiration pattern, increased body mass index and other types of technical faults.
Another tool that has been proposed for the assessment of underlying fibrosis is surface ECG. The more extensive the underlying atrial fibrosis, the slower the conduction within the left atrium, resulting in a prolonged duration of the sinus P wave. Jadidi et al. have reported a correlation between the extent of left atrial low-voltage substrate, which is indicative of underlying fibrosis, and the duration of amplified sinus P wave.19 They also proposed that a cut-off value of 150 msec identifies patients with fibrotic substrate who are also at increased risk of arrhythmia recurrence following catheter ablation of AF and offers high sensitivity and specificity. Thus, this widely available, non-invasive, low-cost tool could be used in everyday practice for the preprocedural assessment of underlying atrial fibrosis content, as well as improve the success rate of invasive arrhythmia management.
Impact on Patient Management
The evaluation of atrial fibrosis as the main indicator of underlying structural remodelling may affect decision making at several stages of the management plan of AF patients. The respective AF treatment domains that are influenced by the presence of underlying atrial fibrosis are assessment of stroke risk and need for anticoagulation; decision for rhythm control strategy; and modification of adopted strategy during catheter ablation.
Assessment of Stroke Risk and Need for Anticoagulation
Atrial fibrosis is associated with a worsened prognosis among AF patients. Patients with more severe left atrial LGE are more likely to have a history of stroke and to present with left atrial appendage thrombus or spontaneous echocardiographic contrast in transoesophageal echocardiography.20,21
In an observational study, King et al. demonstrated that left atrial LGE severity, as a marker of fibrotic structural remodelling, is associated with increased risk of major adverse cardiovascular and cerebrovascular events, particularly stroke or transient ischaemic attack.22 Similarly, Müller et al. showed that a left atrial low-voltage area that was evaluated by intraprocedural mapping during AF catheter ablation (bipolar voltage <0.5 mV) was associated with history of stroke and silent cerebral ischaemia.23
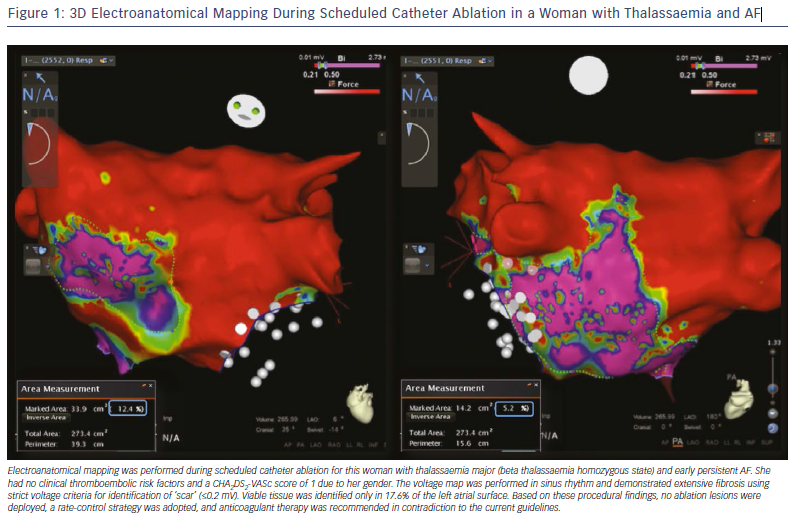
These findings support a potential role of atrial fibrosis in the stratification of ischaemic stroke risk among AF patients. The adopted risk-stratification schemes (CHA2DS2-VASc score) mainly aim to identify patients with a truly low risk score who do not require antithrombotic therapy. Therefore, the presence of atrial fibrosis, especially if severe, may influence decision making in favour of anticoagulation prescription in patients without clinical risk factors who would not otherwise be considered as suitable candidates for anticoagulation protection.24 In the case presented in Figure 1, a young woman with homozygous thalassaemia and paroxysmal AF was prescribed anticoagulant treatment despite the absence of any clinical ischaemic risk factors, and solely based on the extensive atrial fibrosis identified during voltage mapping. However, despite similar anecdotal cases, adequately powered prospective studies are needed to validate the role of atrial fibrosis for guidance of antithrombotic treatment in AF patients.
Decision for Rhythm Control Strategy
The assessment of atrial fibrosis may aid in the selection of patients anticipated to gain benefit from rhythm control management.25 Accumulating data support the notion that the higher the burden of atrial fibrosis, the more complex the underlying mechanism of AF, and thus the more challenging is sinus rhythm maintenance. Jadidi et al. demonstrated that the mean AF cycle length is inversely related to the extent of LGE on CMR.26 Cochet et al. found that left atrial fibrosis, as assessed by LGE, is the only independent predictor of the number of re-entrant regions and the complexity of underlying re-entrant activity in the left atrium among persistent AF patients subjected to high-resolution electrocardiographic imaging.27 These findings are consistent with evidence derived from experimental studies.28,29
Furthermore, in the clinical context, the amount of preablation atrial fibrosis, as estimated by LGE-MRI, is independently associated with the likelihood of arrhythmia recurrence.9,27 The left atrial wall structural remodelling stage (Utah stage) is the strongest predictor of ablation outcome in multivariate analyses.10 Therefore, especially in the case of persistent and long-term persistent AF, a large volume of atrial fibrosis evidenced by LGE-MRI may serve as a gatekeeper to rule out patients from undergoing one or more demanding ablations in the challenging pursuit of sinus rhythm maintenance.
Impact on Ablation Strategy
A wide circumferential electrical isolation of pulmonary veins remains the cornerstone of AF ablation.30 However, despite the need for an adjunctive ablation strategy in addition to pulmonary vein isolation to improve the low rates of sinus rhythm maintenance in patients with persistent and long-standing-persistent AF, randomised clinical trials have failed to show an additional benefit from linear lesions and ablation of complex fractionated electrograms.31
Atrial fibrosis is an attractive target for ablation among patients with AF, as it has been proposed to participate in the complex interplay of diverse pathophysiological mechanisms.32 Despite an existing contradiction on potential spatial correlation between re-entrant activity and underlying atrial fibrosis in AF,27,33 several computational, experimental and human studies have supported the role of atrial fibrosis in anchoring re-entry during AF.34,35 The evident link between fibrotic substrate and re-entrant drivers perpetuating AF has rendered these late gadolinium enhanced areas as potential ablation targets, lending support to the development of an adjunctive ablation strategy to be implemented in addition to pulmonary vein isolation.
The first step in the incorporation of atrial fibrosis as an ablation target during invasive management of AF is the accurate identification of atrial fibrotic areas during voltage mapping. Several studies have shown a spatial correlation between low-voltage areas detected by intraprocedural voltage mapping and fibrotic areas identified by delayed enhancement cardiac MRI.36 The evolution of 3D electroanatomic mapping tools and high-density voltage mapping improved the identification of pathological tissue.37 However, voltage mapping during AF tends to overestimate the extent of underlying atrial fibrosis due to significant differences in electrogram voltage amplitude between sinus rhythm and AF at the same sites among persistent AF patients.38 The optimal cut-off value for accurate demarcation of left atrial scar displays regional variation, and a bipolar voltage of 0.27 mV best identified atrial scar compared with delayed enhancement cardiac MRI.39
The optimal ablation method of atrial fibrotic areas in the context of AF ablation has not been clarified. Several ablation strategies targeting atrial fibrotic areas have been proposed in the context of persistent AF ablation.
Box Isolation of the Fibrotic Area
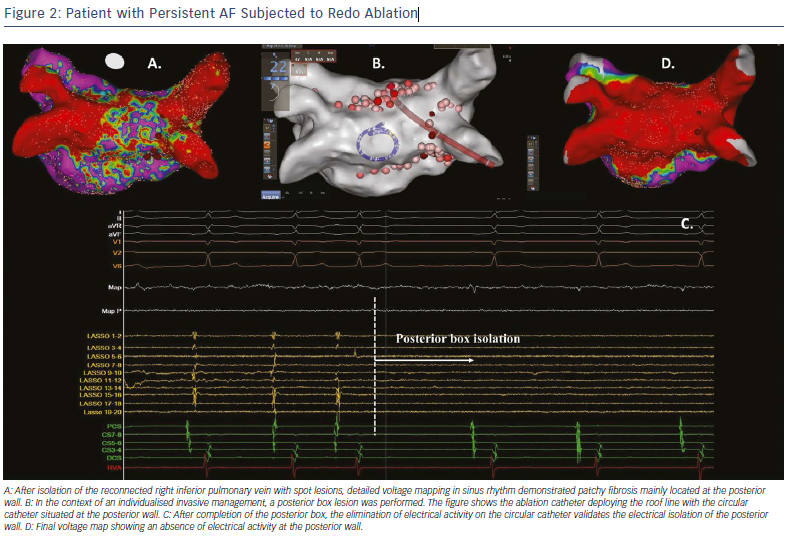
The isolated fibrotic areas should also be connected to neighbouring anchoring lines, such as the circumferential pulmonary vein isolation lines or other empirical lines, including the roof line, mitral isthmus line or anterior line.40,41 The deployed connection lesions aim to prevent micro or macro re-entrant arrhythmias through iatrogenically formed conduction channels around the isolated fibrotic areas. This strategy of individualised substrate modification has been reported to improve the ablation success rate when applied in addition to pulmonary vein isolation during ablation of paroxysmal and non-paroxysmal AF (Figure 2).42
Homogenisation of the Low-voltage Area
Another ablation approach for the management of underlying atrial fibrosis is the homogenisation of low-voltage areas. This method aims to eliminate all detectable electrograms within the borders of the low-voltage area. In contradiction to the clear procedural endpoint of the BIFA strategy, the homogenisation method aims to reduce the amplitude of the recorded bipolar electrograms with diverse criteria (less than 0.1 mV, or more than 50% compared with baseline).43,44 The homogenisation lesions within the fibrotic area are complemented by deployment of linear lesions to eliminate conductions channels that increase the likelihood of iatrogenic re-entrant arrhythmias. Yamaguchi et al. reported that implementation of the homogenisation strategy in addition to pulmonary vein isolation in patients with persistent AF and underlying atrial fibrosis significantly increases the likelihood of sinus rhythm maintenance compared with pulmonary vein isolation alone.44
Selective Ablation of Atrial Low-voltage Sites
A caveat of targeting all atrial fibrotic areas during substrate modification in persistent AF is the lack of specificity in identifying underlying driver re-entrant circuits or rotors.27 In other words, the majority of pathophysiological culprit re-entrant regions are located within atrial fibrotic areas. However, several fibrotic areas do not home re-entrant circuits, and thus their ablation would not be expected to have an impact on AF organisation or termination.27 Therefore, it seems intriguing to select the low-voltage areas to be targeted during ablation based on the presence of certain electrogram criteria suggestive of a critical role in arrhythmia perpetuation. In this concept, the combined implementation of selection criteria based on both indices of electrical remodelling (specific activation patterns) and structural remodelling (low-voltage areas suggestive of fibrosis) aim to increase the specificity of localising target sites for ablation.
Several groups have proposed similar strategies of selective ablation of low-voltage atrial regions. Jadidi et al. identified low-voltage areas of interest for subsequent ablation based on specific regional activation patterns including repetitive presence of prolonged fractionation (>70% of AF cycle length), repetitive rotational activity or discrete rapid activity.45 The same group demonstrated that the ablation of those sites in addition to pulmonary vein isolation was associated with significantly reduced recurrence of atrial tachyarrhythmias compared with pulmonary vein isolation only.45 Furthermore, targeted ablation of specific electrograms in low-voltage areas in addition to wide antral circumferential ablation in patients with persistent AF has been reported to improve patient outcome.46
Other Ablation Strategies Targeting Low-Voltage Areas
A personalised substrate-modification method targeting left atrial low-voltage areas combining several of the elements described in the aforementioned strategies has been reported. Rolf et al. targeted low-voltage areas for substrate modification by homogenisation of the respective area, or deployment of strategic linear lesions either to encircle and electrically isolate large low-voltage areas or to connect these areas with non-conducting structures.47 The combined application of this individualised substrate modification with pulmonary vein isolation significantly increased AF-free survival compared with pulmonary vein isolation alone.
In the same context, the multicentre, randomised Electrophysiological Substrate Ablation in the Left Atrium During Sinus Rhythm (STABLE-SR) trial evaluated the safety and efficacy of a substrate-modification strategy targeting the fibrotic areas in patients with non-paroxysmal AF, with a procedural aim of total tissue homogenisation in low-voltage zones (0.1–0.4 mV), complex electrogram elimination in the transitional zones and de-channelling if considered necessary.48 This strategy resulted in similar rates of arrhythmia-free survival compared with the typical stepwise ablation approach, without, however, implementing any additional substrate ablation in addition to pulmonary vein isolation in more than 50% of patients. These findings further support the concept of an individualised substrate-modification approach tailored to the specific left atrial tissue characteristics of each patient, avoiding potential unnecessary ablation and enhancing procedural safety.
Conclusion
The role of atrial fibrosis in the maintenance of persistent AF is well established. Despite our progress in accurately identifying the presence, location and extent of atrial fibrosis, there are gaps in understanding the optimal way of targeting those fibrotic areas of interest during catheter ablation. Diverse existing ablation strategies aiming to modify the arrhythmogenic substrate and to improve the outcome of patients with persistent AF subjected to catheter ablation need to be tested in adequately powered prospective, multicentre studies. These findings are expected to pave the way towards more effective invasive management of AF.
Clinical Perspective
- Left atrial fibrosis is independently associated with history of stroke and is a risk factor for future thromboembolic events. Evaluation of atrial fibrosis might improve thromboembolic
- Substrate-modification strategies targeting low-voltage areas in the left atrium may improve the long-term outcome of AF ablation.