This document has been approved by the Council of the British Heart Rhythm Society (BHRS) and is a thorough update. This document will be reviewed by the BHRS bi-annually. The purpose of the document is to facilitate the safe delivery of high quality, evidence-based, cardiac device therapy to all patients who may benefit. This includes identification of patients with device indications, implantation of appropriate devices and patient and device follow-up, along with data collection, storage and submission. It is recognised that competence can only be defined effectively in terms of patient outcome. Numbers given in this document are indicative and should not be taken in isolation as evidence of competence or the ability to provide a safe, high-quality service. This document is not intended to disrupt or disenfranchise existing, successful device services.
Definitions
The cardiac rhythm management (CRM) devices within the scope of this document include: permanent pacemakers implanted for bradycardia indications, leadless pacemakers; CRT devices; ICDs implanted for tachycardia (with or without bradycardia indications) and subcutaneous ICDs (SICDs).
For the purposes of this document, in terms of counting cases, an implanter (operator) is defined as the clinician who is either the main individual performing the case or is present throughout the procedure and plays an active part in the procedure. This does not include merely observing the case or offering advice from within or outside the operating room.
Treatment Indications
CRM devices are effective at improving quality of life and reducing mortality. It is essential that all implanting centres submit to the National CRM database.
For ICD and CRT implantation, the development of a multidisciplinary approach to patient selection, management and follow-up is recommended.
Out-of-hours Bradyarrhythmia Emergencies
Patients presenting with bradycardia emergencies, specifically complete heart block, should ideally be directed to a hospital where they can be safely and appropriately managed. Hospitals must have access to facilities or a transfer policy for temporary pacing wires on a 24/7 basis. All patients should be offered early permanent pacemaker implantation. The local cardiac network should oversee regional arrangements.
Requirements for Performing Pacemaker Implantation
Safe device implantation requires the appropriate environment, equipment, trained personnel and culture. This section contains information on these areas. The BHRS endorses and recommends the European Heart Rhythm Association (EHRA) expert consensus statement and practical guide on optimal implantation technique for conventional pacemakers and ICDs, endorsed by the Heart Rhythm Society, the Asia Pacific Heart Rhythm Society and the Latin American Heart Rhythm Society.1 Proficiency in performing on-site pericardiocentesis is required.
Cardiologists
This section refers to cardiologists implanting pacemakers for bradycardia. Implantation of ICDs and CRT devices requires these skills and additional techniques. Complex device procedure numbers (ICD, CRT-pacemaker [CRT-P] and CRT-defibrillator [CRT-D]) can be included with bradycardia pacemaker numbers for the purposes of this section.
- There should be a minimum of two active implanting consultant cardiologists per centre.2
- Each implanter should perform a minimum of 35 new pacemaker implants per year.
- Each implanter should have had appropriate training in pacemaker implantation either as a specialist trainee (ST) or in a suitable alternative training post and reassessment/re-training as a consultant (with assessment of competency) if the recommended implantation numbers have not been performed in the last 12 months.
- If an implanter does not perform this number of procedures in a 12-month period then competence should be independently assessed. Specialist training guidelines are available to guide competency assessment using a structured report for competency ‘sign off.’ This report should include the number of cases performed and the level of competency as assessed by the trainer/assessor. Assessment should consist of successful completion of six directly observed procedural skills (DOPS) in pacemaker implantation at level 3 by at least two assessors.
- At least one implanter should have professional accreditation in device therapy (BHRS, EHRA or International Board of Heart Rhythm Examiners [IBHRE]).
- All implanters must be competent in pacemaker follow-up.
- All implanters must undertake appropriate continuing professional development in device therapy, including implications for driving.
- All implanters must audit their personal complications and share these within their centre and through the National CRM database for clinical governance purposes. If an implanter’s complications were to exceed accepted limits, practice should be reviewed and advice sought from regional device leads or the BHRS. Operators implanting <50 new pacemakers per year may need to average their figures over ≥2 years to account for random variation.
Centres
- Each centre should perform a minimum of 80 new pacemaker implants per year.
- Centres training an ST should be performing more than 100 new implants per year.
- In exceptional circumstances, a centre may not be able to meet these minimum numbers but each individual member of the team undertaking the procedures meets the training and competency requirements plus the individual numbers of implants. The rationale for such a service should be clearly justified; the service must meet local clinical governance requirements including documented protocols to deal with complications and must be robustly audited in terms of quality and outcomes with regular external peer review by the local cardiac network (minimum every 2 years).
- Implantation should be performed in an environment appropriate for aseptic procedures. Standard WHO and National Institute for Health and Care Excellence guidance for preventing surgical site infection should be followed.3,4 Wearing indicator gloves under plain gloves helps identify glove perforation.5
- Standardised hospital guidance on antiplatelet (ideally avoid) and anticoagulant medications should be used and electrocautery should be available.1
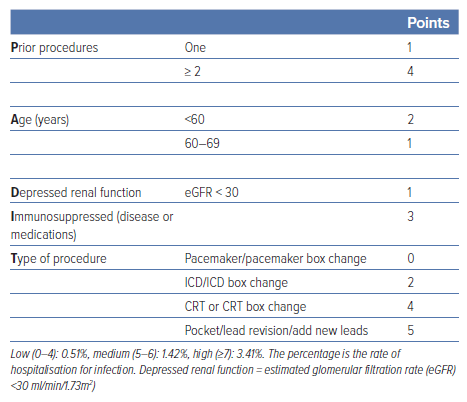
- Selection criteria for antibiotic pouches should be considered for patients who are at higher risk of infection (e.g. BLISTER score ≥6), and the PADIT score (Table 1) is also helpful to identify those at higher risk of infection.6
- The antimicrobial agent tauroline has been used in patients at high risk of infection; registry data show encouraging results following its use associated with a reduced risk of infection in a prospective observational study.7 Chlorhexidine pocket lavage in high-risk procedures was associated with a reduced risk of infection without adverse events in a non-randomised series.8
- All equipment for implantation and management of possible complications must be immediately available, including external defibrillation with external pacing facilities and on-site proficiency in pericardiocentesis.
- Appropriately trained cardiac physiologists/scientists, nurses and radiographers should be present as dictated by local arrangements.
- Each centre should maintain a well-managed database of device activity within the hospital IT infrastructure to allow immediate tracing of patients with device advisories and timely electronic submission of data to the National CRM database.
Each centre must maintain a database of complications to facilitate clinical governance, which must be submitted to the National CRM database. Specific complications are:
- pneumothorax requiring intervention or delay to discharge;
- haematoma requiring intervention;
- pericardial effusion requiring intervention;
- any re-intervention within 12 months from implant.
Complications should be recorded at discharge and at 12 months’ (9– 15 months’) follow-up. Complications should be tracked by National Health Service number by the National Institute for Cardiovascular Outcomes Research (NICOR).
- Each device centre must ensure that they have agreements and arrangements in place that allow their patients access to MRI scanning. m. Extraction referral pathways supported by the local cardiac network should ensure timely extraction is available in patients with infected devices.
- Extraction referral pathways supported by the local cardiac network should ensure timely extraction is available in patients with infected devices.
Physiologists/scientists
- There should be at least two cardiac physiologists/scientists actively involved in pacemaker implantation and follow-up in each centre.
- Each physiologist/scientist must have had appropriate training in pacemaker implantation and follow-up.
- At least one physiologist/scientist should have a current certification in device therapy (BHRS, EHRA or IBHRE).
- All physiologists/scientists must undertake appropriate continuing professional development in device therapy and associated patient advice, including implications for driving according to Driver and Vehicle Licensing Agency (DVLA) guidelines(e.g. time post-implant, relationship to device therapies, impact of cardiac function, etc.).
- Each physiologist/scientist should be actively involved in a minimum of 35 new pacemaker implants per year. If a physiologist/scientist does not perform this number of procedures in a 12-month period, then competence should be independently reassessed. If the physiologist/scientist is also undertaking follow-up of complex devices (ICD and CRT-D/-P), the implant numbers can include these devices.
Nurses
- At least one of the cardiac arrhythmia nurses involved in device management should have current accreditation in device therapy (BHRS, EHRA or IBHRE).
- Cardiac arrhythmia nurses involved in device therapy must undertake appropriate continuing professional development in device therapy and associated patient advice including implications for driving according to DVLA guidelines (e.g. time post-implant, relationship to device therapies, impact of cardiac function, etc.).
Leadless Pacing
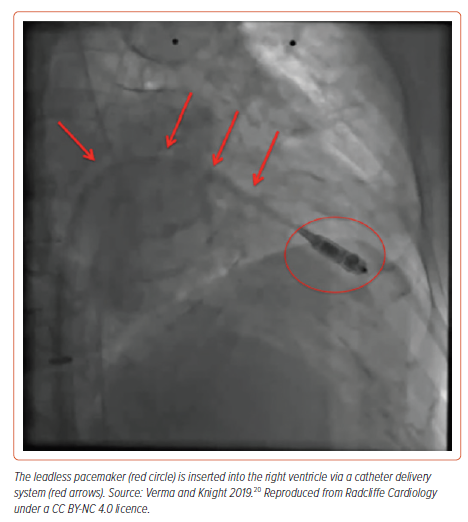
Leadless pacing is an option for patients requiring bradycardia pacing, and some systems can also provide ATP in combination with an SICD (Figure 1). A UK expert consensus statement noted that leadless pacing is regarded as safe and an effective alternative to conventional transvenous pacing.9 Leadless pacing should take place in a cardiac cath lab with access to pericardiocentesis.10 Shared decision-making should be undertaken, mentioning the risk of serious acute events including tamponade requiring urgent thoracotomy, device displacement and vascular access issues. There should be careful attention to contraindications such as patient habitus and venous abnormalities that could present problems with access and complications from the large sheaths required. Ultrasound-guided venous access is recommended. In the UK, specific guidance is available.10 UK centres should develop a hub and spoke arrangement if they do not provide implantation locally; followup should be local and any complications should be notified to the implanting centre and the Medicines and Healthcare products Regulatory Agency (MHRA) (device-related). Appropriate training of new operators is essential and initial implants should be undertaken while overseen by an experienced operator.
Implants could be considered for the following patients (atrioventricular [AV] synchrony may be important for particular patients, e.g. some adult congenital heart diseases), such as those with
- high risk of infection;
- end-stage renal disease;
- previous device infection;
- anatomical constraints complicating/precluding transvenous pacing;
- immunocompromised;
- biological therapies (including immunosuppressants and corticosteroids);
- undergoing radiotherapy where the device is close to site of radiotherapy;
- high probability of needing indwelling vascular catheters.
Implants could be considered for the following groups, noting that AV synchrony may be important:
- congenital heart disease;
- aged <40 years.
Additional Requirements for Performing ICD and/or CRT-defibrillator/CRT-pacemaker Device Implantation (Complex Devices)
Implantation of ICDs and CRT devices carries higher immediate and longterm complication rates and potential rates than bradycardia pacemakers. Awareness of the futility of ICD therapy is important in patients with multiple comorbidities; the Charlson Comorbidity Index may be helpful in guiding discussions.11
All the above standards relating to pacemakers, in terms of the number of cardiologists, physiologists/scientists, etc. need to be met in addition to the following requirements:
Cardiologists
- There should be a minimum of two active implanting ICD/CRT consultant cardiologists per centre.2
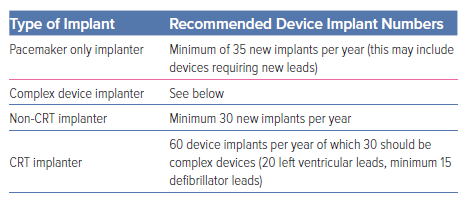
- Each implanter should perform a minimum of 30 new complex device implants or upgrades per year. As the skills necessary for CRT are different from those required for ICD implantation, if an operator is implanting CRT devices, at least 20 of the leads implanted should be left-ventricular leads, and 15 defibrillator leads. The BHRS does not wish this guidance to prevent appropriate patient care. Some electrophysiologists, who have implanted high numbers of devices and meet the minimum complex ablation standards, undertake a wide range of interventional electrophysiology procedures requiring on-going skills in wire and catheter manipulation. There are circumstances in which electrophysiologists are required to undertake complex devices because of service demands, including device implants when on call, covering sickness and many devices are extracted by electrophysiologists with follow-on lead implantation. It is important that the local centre ensures devices implanted are inserted and programmed appropriately (Table 2).
- Each implanter should have had appropriate training in ICD and CRT implantation, either as an ST or in a suitable alternative training post, and reassessment/re-training as a consultant if the recommended implantation numbers have not been performed in the last 12 months.2
- If an implanter does not perform this number of procedures in a 12-month period then competence should be independently assessed in accordance with training guidelines.2 A structured report should be obtained from the trainer/assessor on competency for ‘sign off’. This report should include the number of cases performed and the level of competency as assessed by the trainer/assessor. Assessment should consist of successful completion of six DOPS in CRT implantation at level 3 by at least two assessors and/or six DOPS in ICD implantation at level 3 by at least two assessors.
- Those in training and consultant implanters must be performing a minimum of 30 new ICD or CRT implants or upgrades per year (40 is desirable).
- All implanters must be fully competent in ICD and CRT follow-up.
- All implanters must undertake appropriate continuing professional development in ICD and CRT therapyincluding implications for driving.
Centres
- All patients being considered for ICD/CRT implantation should be fully assessed to determine the aetiology of their cardiac dysfunction or primary arrhythmic condition.
- Patients surviving cardiac arrest or sustained ventricular tachycardia require access to assessment by appropriately trained cardiologists with expertise in defibrillators and knowledge of electrophysiology.
- Each centre should perform a minimum of 60 new ICD or CRT implants per year.
Physiologists/scientists
- There should be at least two cardiac physiologists/scientists actively involved in ICD/CRT implantation and follow-up in each centre.2
- For ICD/CRT implantation, physiologists/scientists should have documented experience of a minimum of 25 new ICD implants and 25 new CRT implants performed under supervision and experience of at least 25 ICD and 25 CRT follow-up evaluations.
- Each physiologist/scientist should be actively involved in a minimum of 30 new ICD/CRT implants or upgrades per year.2 If a physiologist/ scientist does not perform this number of procedures in a 12-month period, then competence should be independently reassessed.
- All cardiac physiologists/scientists involved in ICD/CRT must be fully competent in ICD and CRT follow-up and professionally accredited.
- All cardiac physiologists/scientists involved in ICD/CRT must undertake appropriate continuing professional development in ICD and CRT therapy including implications for driving.
- Device programming should be standardised as per international guidance.
Subcutaneous Defibrillators
SICDs are used routinely in current practice (Figure 2). The BHRS recommends they are considered in selected patients needing defibrillators. Developments in the technology include leadless modular right-ventricular pacing devices to provide anti-tachycardia (ATP) and bradycardia pacing, and a sub-xiphisternal approach that can deliver ATP in a single device. Centres undertaking SICD should maintain a reasonable volume, suggested as more than 10 cases per year, undertake appropriate pre-device screening and have facilities to provide patient comfort options such as deep sedation, general anaesthesia and nerve blocks. In patients who need programmed ventricular tachycardia induction, appropriate resuscitation capabilities should be in place, a time out should be undertaken prior to induction and a failed shock (failure to successfully defibrillate) protocol should be available. Complication data must be submitted to the national audit and appropriate local governance arrangements should be in place.
SICD is considered as a first choice in:
- paediatric or adult congenital heart disease patients with no venous access;
- patients with acquired stenosis or obstruction of central veins unless fibroplasty is available;
- patients with previous endocarditis or device infection;
- patients at very high risk of infection of endovascular leads, e.g. dialysis, immunodeficiencies, cancer, need for a chronic indwelling catheter;
- candidates for cardiac transplantation.
SICD is a reasonable choice in:
- young patients with an active lifestyle and a long life expectancy;
- patients with inherited genetic arrhythmogenic syndromes (Brugada syndrome, long and short QT, early repolarisation);
- patients with hypertrophic cardiomyopathy;
- patients with prosthetic heart valves (infection risk);
- women (may be preferred as potentially less body distorting);
- primary prevention patients with ischaemic/non ischemic dilated cardiomyopathy;
- secondary prevention patients/survivors of out-of-hospital ventricular fibrillation.
The SICD should be avoided if there is:
- failed pre-implant screening (7–11% of cases);12
- symptomatic bradycardia requiring permanent pacing;
- previously implanted unipolar pacemaker (sensing/detection pitfalls);
- systolic heart failure and left bundle branch block, with an indication for CRT;
- recurrent sustained monomorphic ventricular tachycardia treatable with ATP (unless SICD or module able to deliver ATP);
- anatomical characteristics such as thin patients with poor subcutaneous tissue or pectus excavatum.
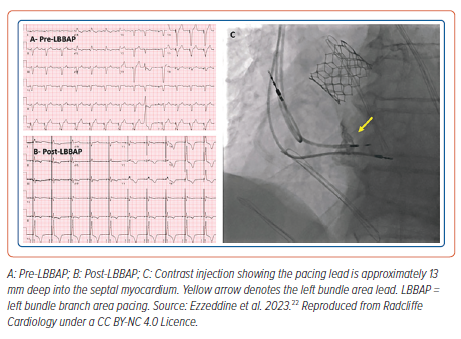
Conduction System Pacing
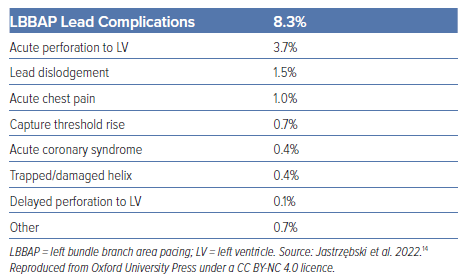
Conduction system pacing (CSP) encompasses His bundle pacing (HBP) and left bundle branch area pacing (LBBAP). CSP may be considered in a wide range of patients as detailed in international guidelines (Figure 3).13 In terms of implantation, CSP requires knowledge of electrophysiology and a team of physiologists and implanters dedicated to obtaining good results. There is a learning curve to the techniques. Centres should be aware of the risk of coronary artery perforation and associated septal haematoma with deep septal pacing (Table 3).14 Meticulous attention to unipolar (tip to tissue) lead impedance, evoked electrogram potentials and paced electrogram morphology is important at the time of implantation. The paced electrogram current of injury may be abnormal in patients with septal perforation. It is important to confirm visually the position of lead in the septum in the left anterior oblique position using fluoroscopy. Physiologists/scientists aid the implant by monitoring lead progress and CSP capture during the implant.
His bundle pacemaker follow-up requires detailed and meticulous attention to detail to pick up septal pacing, rising thresholds particularly in pacing-dependent patients or those where CSP is being used in lieu of CRT. HBP can be associated with rising pacing thresholds, failure to capture during follow-up, small sensed R waves and the risk of P-wave sensing.
Centres considering starting CSP programmes outside of research trials should acquire appropriate training to be able to safely undertake initial deep septal implantation for left bundle area pacing (such as using a proctor). It is recommended that implant success, complications and initial follow-up are audited. Manufacturer- and lead-specific guidance should be followed. A unipolar myocardial pattern of injury is helpful to detect perforation.
Recommended Knowledge
- Implanter: safe implant technique, awareness of lead behaviour during implantation, unipolar high rate ventricular pacing, effect of programmed stimulation.13 LBBAP: awareness of fluoroscopy projections needed, unipolar pace mapping, unipolar impedance safety, unipolar paced evoked potential, unipolar morphology of a successful implant, unipolar left ventricular activation time (LVAT), unipolar V6 to R wave V1 interpeak time, QRS duration, ST segment elevation, left bundle potential to QRS time, fixation beats (skinny ectopics). HBP: non-selective, selective and septal unipolar pacing, H to V time and recognition of prolonged time. Device selection and programming.
- Physiologist/scientist: recommended to have completed specific continuing professional development for implantation and follow-up. Unipolar high-rate ventricular pacing, programmed electrical stimulation technique, 12 lead ECG. Projecting electrophysiology and pacing screen, set up of filters, unipolar pace mapping, unipolar impedance safety, unipolar paced evoked potential, unipolar morphology of a successful implant, unipolar LVAT, unipolar V6 to V1R time, QRS duration, ST segment elevation, left bundle potential to QRS time, fixation beats (skinny ectopics).13 HBP: non-selective, selective and septal unipolar pacing, H to V time and recognition of prolonged time. Device selection and programming.
Essential Equipment
- 12-lead ECG machine.
- Fluoroscopy.
Recommended Equipment
- Electrophysiology system.
- Slave display of device programmer.
Recommended Documentation
- NICOR and local audit.
- 12-lead paced ECG.
- Left-ventricular activation time, QRS duration, V6 to V1 interpeak time, pacing impedance, R wave unipolar, R wave bipolar, R wave morphology unipolar.
Requirements for Cardiac Rhythm Management Device Follow-up
Device follow-up clinics now encompass many types of CRM devices. Follow-up of devices involves knowledge of other features such as the detection of heart failure and the ability to deactivate ICDs and program for MRI scanning. It is important that appropriate levels of training are in place and that clinical governance and lines of clinical responsibility are clearly established for all follow-up procedures.
The objective of device follow-up is to maximise the health outcomes and wellbeing of patients by programming devices to deliver the most effective evidence-based therapy, by promptly identifying and minimising system complications and using device diagnostic data to guide treatment. Device follow-up remains the ultimate clinical responsibility of the Trust, with a named consultant cardiologist leading the device service, although it is predominantly a cardiac physiologist/scientist-run service. Physicians providing such a service must have the required knowledge to do so. A minimum of one physician at a follow-up centre should be professionally accredited (BHRS, EHRA or IBHRE).
Detailed guidance for device follow-up is on the BHRS website; however, the general principles are detailed below.
Physiologists/scientists
Device follow-up clinics should be undertaken with a minimum of two physiologists/scientists immediately available, of whom the senior physiologist/scientist must have evidence of postgraduate training in CRM, e.g. current BHRS, EHRA or IBHRE certification, current Immediate Life Support or Advanced Life Support certification and evidence of continued professional development in CRM, with knowledge and skills equivalent to Agenda for Change band 7.
Centres
- Pacemaker follow-up: there should be a clearly defined protocol documenting the lines of communication and support between the lead cardiac physiologist/scientist for a bradycardia pacemaker follow-up service and the consultant cardiologist responsible for the on-site service to ensure that clinical governance requirements are met. The lead physiologist/scientist for bradycardia pacemaker follow-up services at non-implanting hospitals must also have a clearly defined protocol documenting the lines of communication with the lead physiologist/scientist and consultant cardiologist at the implant centre. Trusts delivering bradycardia pacemaker follow-up services have a responsibility to ensure appropriate arrangements are in place to cover clinic activity (elective or urgent).
- Complex devices: the BHRS recommends that all hospitals have local or network arrangements to manage patients with ICDs who are admitted with multiple shock delivery, non-delivery of appropriate therapy or other device-related issues. Referral pathways should exist for decompensated heart failure, ventricular tachycardia ablation, awareness that ICD shock therapy is one criteria for referral for advanced heart failure treatment in appropriate patients (“I NEED HELP” mnemonic stands for inotropes, NYHA class/naturetic peptides, ejection fraction, defibrillator shocks, hospitalisation, oedema / escalating diuretics, low blood pressure, reduced prognostic medication) and palliative care.15,16
- Remote device follow-up: remote follow-up is encouraged, particularly for complex devices as per international guidelines, and this may be alert-based where the burden of alerts can be pro-actively managed allowing reduction of calendar based follow-up. In-person follow-up will be needed for re-programming. As many devices undertake automated measurements, it is important for physiologists/scientists to maintain skills in device troubleshooting.
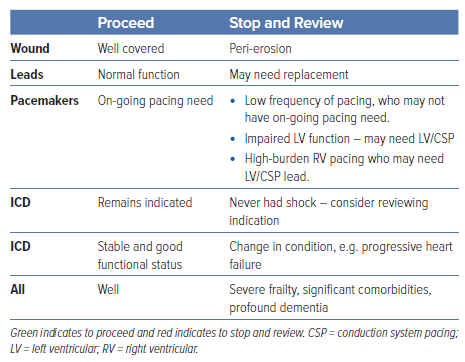
Consideration at Elective Replacement of Pacemaker Generators (Box Changes) and Management of Frailty, Dementia and Life-limiting Conditions with an ICD
The BHRS recommends that patients whose battery is depleting are identified so that informed shared decision-making can occur. Some patients will have straightforward needs for a box change; others may require review to avoid decisions occurring on the day of admission (Table 4).
Prior to first implantation, it is recommended that the patient is aware that defibrillator therapy can be discontinued if their general condition changes, and staff interacting with the patient should be empowered to offer holistic care including broaching discontinuation of shock therapy.
Patients with progressive conditions, e.g. heart failure, those with early dementia and their families should be encouraged to undertake shared decision-making while they have capacity so that plans are in place for when their condition deteriorates, e.g. device therapy discontinuation or not replacing generators. Discontinuation of ICD shocks should be discussed when a patient’s prognosis is likely to be limited or their quality of life/comorbidities change and a shared decision-making approach should be followed. The BHRS recognises this is a difficult area for patients and clinicians alike. The BHRS recognises that replacement of a device generator (including pacemakers for bradycardia) may not be appropriate in patients with severe comorbidities. Patients who develop comorbidities whose ICD battery is depleting should be identified prior to the procedure date, and a discussion should be held around the option of discontinuation or leaving the ICD switched off or changing to pacing only (as appropriate). Patients who are under remote follow-up should be offered the opportunity of contacting the centre to undertake shared decision-making if they feel their condition has changed.
- All device follow-up centres and integrated care boards (including those that only follow up pacemakers) should have a policy in place for discontinuation of ICD function in ICD and CRT-D devices, which should include domiciliary visits when required.
- Shock therapy discontinuation should be a shared decision that includes the treating physician, the patient and – where appropriate – a representative for the patient (e.g. a relative).
- Centres should have a mechanism for ensuring that a patient whose device battery is depleting are not automatically listed for a box change without scrutiny of their comorbidities, on-going device need and underlying tissue.
The BHRS has produced guidance on patient choices and ICD therapy discontinuation. The BHRS recommends the Resuscitation Council UK guidance on ICD therapy discontinuation.17,18 Other guidance is available, for instance guidance from CorHeath Ontario and UK palliative care networks.18,19
Audit
Device therapy is subject to immediate and long-term complications. There are also frequent advice and safety notices from manufacturers and the MHRA that necessitate timely action. All implanting centres must collect data on their patients, devices and follow-up that is immediately available and facilitates audit. All implanting centres must contribute to the National CRM Database, including submission of 12-month follow-up data.
Day-case Elective Device Implantation
Day-case device implantation is encouraged unless overnight admission is required according to local and international guidance. Patients should have a contact telephone number for staff experienced in device complications.