Historical Precedents
Brugada syndrome (BrS) was first described more than 25 years ago as a clinical entity in people resuscitated from sudden cardiac death due to documented VF.1 The original 1992 case series described eight patients without apparent structural heart disease who all had VF associated with persistent coved ST-segment elevation in the right precordial leads.1 In 1996 this arrhythmic syndrome was named Brugada syndrome. The next year, BrS was recognised as the same clinical entity as sudden unexplained nocturnal death syndrome, first reported in 1917 in the Philippines.2 The syndrome was considered a familial disease because of syncope and/or sudden death in many relatives of a same family, and the first genetic alteration was identified in 1998.3
Typical presentation of the syndrome is syncope or resuscitated sudden death, and symptoms usually occur at night or at rest especially after a large meal. Fever is a common trigger, particularly in children. As subsequent registry data were published, it became apparent that the spectrum of risk is wide, with most patients classified as low risk. Despite intense research efforts, as documented by about 5,000 publications on BrS, controversies still exist over its pathophysiology, risk stratification and care. In the last 20 years, 12-lead surface ECG has represented the primary source of information for diagnosis and prognosis, but the specificity and accuracy of the abnormal ECG pattern are relatively low.4
Brugada Syndrome Burden
At present, it is challenging to establish the actual burden of the syndrome, mainly because we do not know the real number of asymptomatic people due to the high variability and fluctuations of the typical ECG pattern. The incidence appears to be low (<1%), but the condition is responsible for >10% of all sudden deaths and up to 20% of sudden deaths in structurally normal hearts. The prevalence is 8–10 times higher in men than women. More data are becoming available about unexpected deaths in different populations, so the real incidence of BrS needs to be updated.5
Clinical Presentation
The most typical presentation of BrS is syncope or resuscitated cardiac arrest in the third or fourth decade of life due to polymorphic ventricular tachycardia (VT) or VF. Symptoms typically occur at night or at rest during the day, and also uncommonly during exercise.
Monomorphic VT is rare and is more prevalent in children and infants, for whom fever is the most common trigger. Diagnosis may also be made on familial screening of patients with BrS or incidentally following a routine ECG.
Symptoms typically first develop during adulthood, commonly at 40 years, but they may occur also in children or older people. More than 80% of adult patients are men, but there is an equal male:female ratio in children. However, the clinical presentation of BrS has changed.6 In more recently diagnosed patients, there has been a decrease in resuscitated cardiac arrest as the first clinical presentation of the disease, thereby making inducibility and risk stratification crucial.6 Many people will remain asymptomatic throughout their life.
ECG Pattern
In 2012, an expert consensus panel clarified ECG characteristics and diagnostic criteria and established two ECG patterns for BrS.7 Type 1 (coved-type) represents the only diagnostic pattern for BrS, while type 2 (saddle-back type) is only suggestive of BrS. The type 2 pattern is characterised by an ST-segment elevation >0.5 mm (usually >2 mm in V2) in >1 right precordial lead (V1–V3) followed by a convex ST.
To facilitate differentiation of type 2 ECG from other Brugada-like patterns, additional criteria have been suggested that utilise the triangle formed by the ascending and descending branch of the R-wave.8 Frequent day-by-day fluctuations in the ECG pattern may occur in the same patient, including a normal pattern (concealed BrS).9 Placement of the right precordial leads in more cranial positions can increase sensitivity due to variable anatomical correlation between the right ventricular outflow tract (RVOT) and V1–V2 in the standard position. Abnormal ECG intervals including P wave duration, PR or QRS duration may be commonly observed. In up to 20% of patients, AF or supraventricular tachycardia due to atrioventricular (AV) nodal re-entry or Wolff-Parkinson-White syndrome have been reported.10
Pharmacological Testing
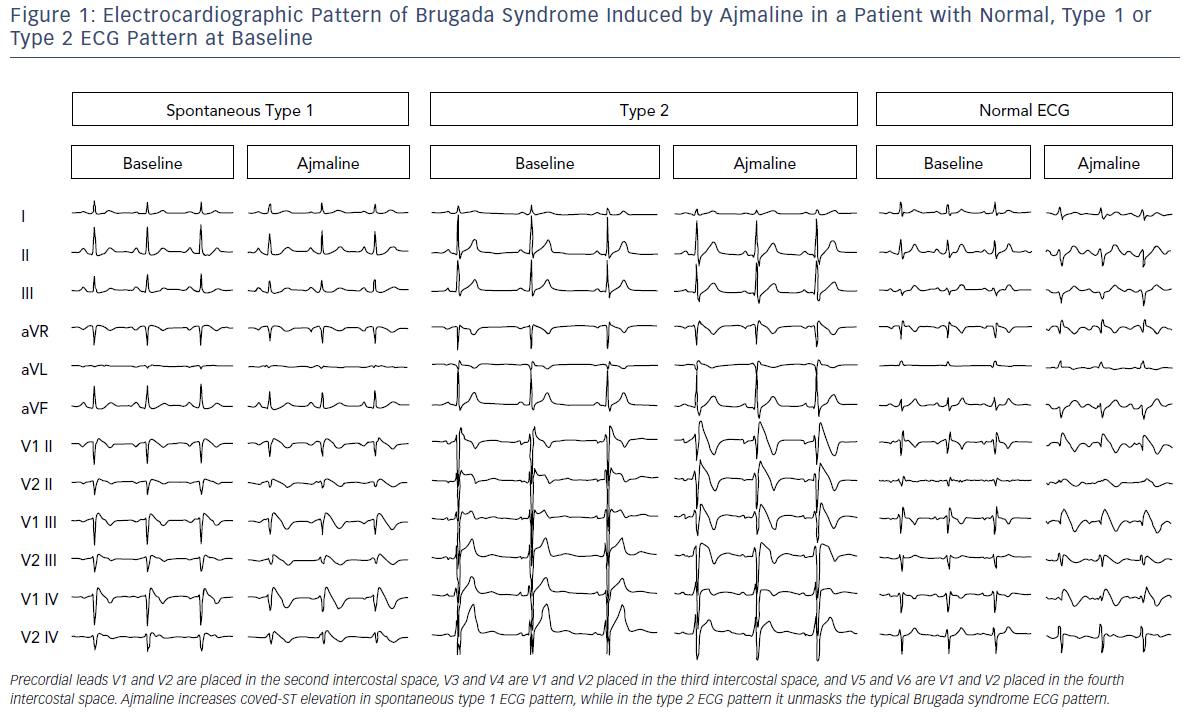
Further investigation is needed in cases where there is a suspicion of BrS (syncope, dizziness, agonal respiration, resuscitated cardiac arrest, family history of BrS or suggestive ECG pattern) but patients do not have a spontaneous type 1 ECG pattern. They should have a pharmacological test performed with a sodium-channel blocking drug under continuous monitoring.11 The test is positive when a type 1 ECG pattern appears during infusion (Figure 1). In the presence of QRS widening (>130%) or the occurrence of frequent premature ventricular contractions (PVCs) or complex ventricular arrhythmias, pharmacological testing should be stopped.11
It should be emphasised that about a quarter of tests may deliver a false negative. This is important when evaluating a patient who has experienced a frank syncope or an aborted sudden death. Ajmaline is the ideal drug for this purpose because of its shorter duration of action (1 mg/kg over 10 minutes, maximum 100 mg; Figure 1); and higher sensitivity than flecainide, but it is not available in many countries. The IV formulation of flecainide (2 mg/kg over 10 minutes, maximum of 150 mg), is not always available in many countries in IV formulation although it is generally available as an oral formulation.
A contraindication to pharmacological testing is PR prolongation in the baseline ECG because of the risk of inducing AV block. A drug challenge should be performed under strict monitoring of blood pressure and 12-lead ECG, and facilities for cardioversion and resuscitation should be available. Moving leads V1–V3 up to the second intercostal space improves diagnostic yield. The patient needs to be monitored for 3 hours or until the ECG is normalised as late positive tests have been reported. The plasma half-life of flecainide is 20 hours, while ajmaline is 5 minutes. Isoprenaline infusion may be employed to counteract these drugs if serious ventricular arrhythmias develop.
Genetic Basis
The first genetic alteration in BrS was identified in 1998 in the SCN5A gene by Chen et al.3 The current challenge in clinical genetics is the interpretation of genetic alterations and their translation into clinical practice.12 To date, nearly a quarter of BrS patients were found to be carriers of SCN5A variants. Over 300 SCN5A variants have been found to be associated with BrS, the majority located in SCN5A, but the causal role of these mutations in BrS is not always clear. Even within families, the observed phenotypes carrying the same SCN5A variant are highly diverse. Environmental and epigenetic alterations also determine variable disease severity. However, the high number of variants may be an overestimate, according to recent guidelines from the American College of Medical Genetics.12
ECG findings predictive of SCN5A mutations include longer and progressive conduction delays (PQ, QRS and HV intervals). The degree of ST elevation and the occurrence of arrhythmias do not differ between subjects with and without an SCN5A mutation.12 Therefore, the presence or absence of an SCN5A mutation does not have any effect on the incidence of sudden cardiac death in BrS. It should be emphasised that BrS is not the only condition attributed to SCN5A mutations. It is well-known that long QT syndrome type 3, progressive cardiac conduction disease (Lenegre’s disease), idiopathic VF, sick sinus syndrome, dilated cardiomyopathy and familial AF are all linked to SCN5A mutations and overlapping syndromes have been reported.12
BrS is commonly accepted as an autosomal dominant channelopathy, however, recent data suggest that it follows a more complex polygenic inheritance model.12 BrS can result from the presence of several variants that confer susceptibility to the phenotype in a given person. At present, genetic analysis in BrS has little to contribute to diagnosis, prognosis and therapeutic management, in contrast to long QT syndrome type 3.12 It does not yet appear to play an important role in risk stratification. As a result, further large studies are required to clarify the exact role of novel genetic variants in BrS pathogenicity for potential therapeutic strategies.
Diagnostic Criteria
Many patients with type 1 ECG pattern are asymptomatic. Therefore, the 2015 European Society of Cardiology (ESC) guidelines proposed a new diagnosis for BrS.13 This is essentially based on the typical ECG pattern, either spontaneous or after sodium-channel blocker, showing in at least one right precordial lead (V1 and V2) positioned in the second, third or fourth intercostal space, without requiring any evidence of malignant arrhythmia.
This definition was challenged in an expert consensus conference report endorsed by the Heart Rhythm Society, European Heart Rhythm Association, Asia Pacific Heart Rhythm Society and the Latin American Society of Cardiac Pacing and Electrophysiology.4 The task force was concerned that the ESC definition could result in over-diagnosis of BrS, particularly in patients who only display type 1 ECG after a drug challenge. Data suggest this latter group is at very low risk and that the presumed false-positive rate of pharmacological challenge is not trivial.14 ECG should thus be routinely performed when a diagnosis of BrS is suspected but is uncertain on a standard ECG, and in screening family members of BrS patients (Figure 1). Typical ECG changes of BrS can also be brought on following a meal and on standing. Rarely, ST changes of BrS may be detected in inferior or lateral leads.
Other ECG Findings in Brugada Syndrome
In BrS, the PR interval may be increased (≥200 ms), particularly for genetic variants affecting the sodium-channel SCN5A, which frequently reflect the presence of an increased HV interval. Also described are:
- P wave abnormalities (prolonged or biphasic P waves);
- late potentials detected by signal-averaged ECG;
- QRS widening; and
- fragmented QRS.
AF occurs in about 10–20% of BrS patients and is associated with increased risk of syncope and sudden cardiac death. Sick sinus syndrome, neurally mediated syncope and atrial standstill have also been described. Conduction delays in the RVOT have also been reported.
Misdiagnosis of Brugada Syndrome
Diagnosis of BrS requires exclusion of other causes of ST-segment elevation (Brugada phenocopies). It is well-known that spurious BrS type ECG changes can be observed following cardioversion, can last for a few hours and may lead to an incorrect diagnosis of BrS. Misdiagnosis of BrS can occur with:
- ECG changes of early repolarisation;
- athlete’s heart;
- right bundle branch block;
- acute pericarditis;
- MI;
- Prinzmetal angina;
- arrhythmogenic right ventricular cardiomyopathy (ARVC);
- myocarditis;
- Duchenne muscular dystrophy;
- electrolyte disturbances; and
- hypothermia.
As in all cases of Brugada phenocopies, a sodium-channel blocking agent will be usually negative.
Risk Stratification
Asymptomatic people are the majority (about 63%) of newly diagnosed Brugada patients. Although the reported annual rate of asymptomatic BrS events has decreased over time, this is not negligible (0.5%–1.2% annual incidence), leading to a malignant arrhythmic events rate of 12% at 10-year follow-up in a population with a mean age of 40 years.4,5
Unfortunately, for most patients the first symptom is cardiac arrest or sudden cardiac death. Therefore, risk stratification of asymptomatic patients is of utmost importance. Identification and management of asymptomatic subjects at high risk of sudden death represent the major challenges in BrS.4,5,13–15 In cardiac arrest or patients with presumed arrhythmic syncope, these strategies are of little use, since these people are already recognised to be at high risk.
Syncope in combination with a spontaneous type 1 ECG pattern is a universally accepted risk factor because up to 62% of symptomatic BrS patients will experience a new event 48–84 months after diagnosis, leading to sudden death. However, there are no clear-cut recommendations for the asymptomatic group. The recent guidelines neither encourage nor discourage electrophysiological study and VT/VF inducibility patterns for BrS stratification in patients with BrS.13 These recommendations are also supported by several large prospective registries and by a recent pooled individual patient data analysis including eight prospective studies.15
Several non-invasive risk stratification markers have been proposed, including signal-averaged ECGs, but the results derive from small observational studies and require validation in larger series.16
Management of Brugada Syndrome
Management of patients with BrS continues to be challenging. There are limited therapeutic options, essentially ICD implantation and quinidine.13
An ICD is always indicated in symptomatic BrS, i.e. resuscitated cardiac arrest and/or non-vagal syncope, or nocturnal agonal respiration. An electrophysiological study may be performed in asymptomatic patients with spontaneous type 1 ECG to assess the need for an ICD.13–15 Although effective for preventing sudden cardiac death, ICD also carries a relevant risk of complications over the patient’s lifetime, particularly if the patient is younger at the time of device implantation.17 Beyond a high prevalence of inappropriate shocks, ICD implantation at a young age also exposes patients to recurrent risks of infection, secondary to device changes and lead complications, frequently requiring subsequent extraction procedures that carry a risk of death.17 ESC guidelines strongly recommend that all BrS patients should be educated about modulating or precipitating factors and taught to avoid these.13
Quinidine has a high rate of effectiveness in the electrophysiology laboratory and has been used to suppress VF in several clinical scenarios, including arrhythmic storms or multiple ICD shocks, or as an alternative to an ICD in children. Unfortunately, the use of quinidine is limited by its unavailability in many parts of the world and its relatively high incidence of side-effects.
Treatment of Arrhythmic Storms
Isoprenaline infusion is effective in acute situations and quinidine is the only effective drug in long-term treatment. Cilostazol has also been shown to be effective and is recommended for long-term treatment.2
Epicardial Ablation in Brugada Syndrome: Moving from Promise to Reality
Since its introduction in 1992, assessment of BrS has focused on parameters based on the ECG, 24-hour Holter recording, and/or electrophysiological testing. However, in the last 30 years, the spectacular success of RF ablation in eliminating all supraventricular arrhythmias led electrophysiologists to search for arrhythmic substrate sites as a target for catheter ablation in patients with BrS and VF because antiarrhythmic drugs have been ineffective in preventing recurrent VF episodes. The recent discovery using 3D electroanatomical mapping of a well-defined potentially reversible arrhythmic substrate in patients with BrS is one of the new key research areas of the 21st century that will allow moving from promise to reality in the management and care of BrS.18–24
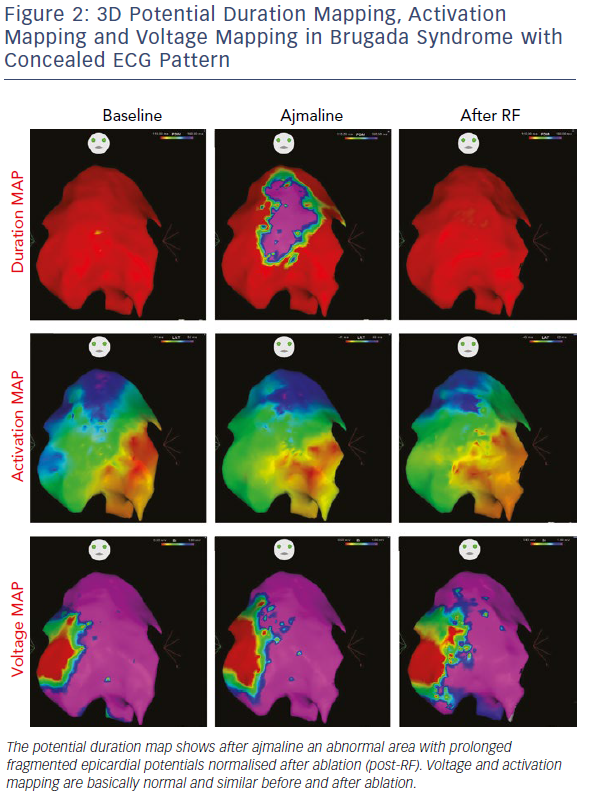
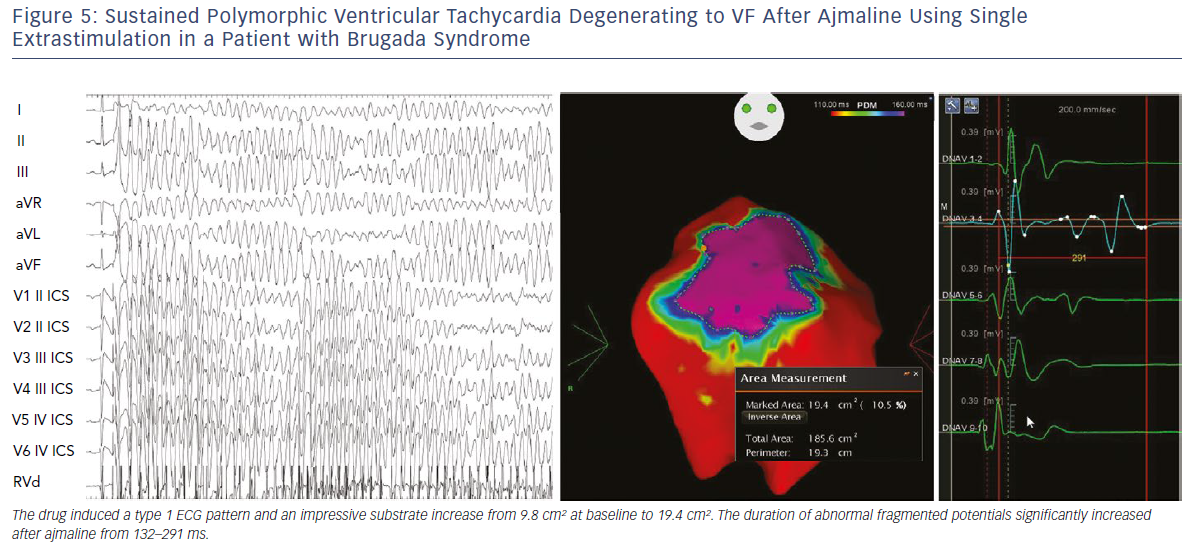
Initial observations by Nademanee et al. in BrS patients with frequent electrical storms proved epicardial ablation to be effective in controlling ventricular arrhythmias during follow-up in eight of the nine patients.18 Subsequently, our group used 3D potential duration mapping by the CARTO system (Biosense Webster) to demonstrate for the first time that in BrS ajmaline was able to reveal highly variable arrhythmogenic substrates, characterised by abnormally prolonged and fragmented epicardial potentials (Figure 2). The substrate size ranged from a small area corresponding to the superior part of RVOT towards an extensive area from the medial to inferior aspect of the anterior RV free-wall without involving other regions of the RV or LV.19–21
Additionally, such abnormal electrograms were only recorded when coved-type ST-segment elevation was present, either spontaneously or after ajmaline provocation. These findings are clinically relevant and suggest that sodium-channel blockade, i.e. ajmaline and flecainide, or warm water instillation into the pericardium can unmask abnormal areas further and increase the size of the VF substrates to be targeted, thus leading to a more successful epicardial ablation that eliminates the Brugada pattern.19–22 Interestingly, many patients with concealed ECG pattern became inducible only after there was a consistent ajmaline-induced increase of the substrate size.21 Re-induction of coved-type ECG pattern by ajmaline after ablation was commonly caused by residual abnormal electrograms in the corresponding epicardial RVOT area.21 By contrast, disappearance of coved-type ECG pattern was due to elimination of the remaining epicardial substrate by catheter ablation.21 The presence of such abnormal potentials can be commonly transient and is correlated with ST-segment elevation and VT/VF inducibility.21
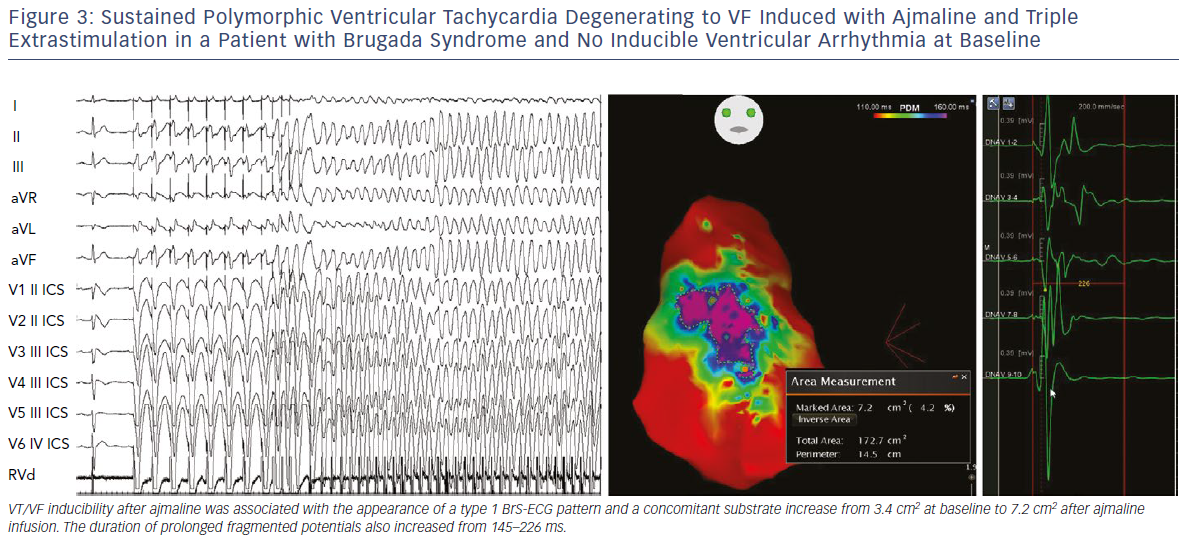
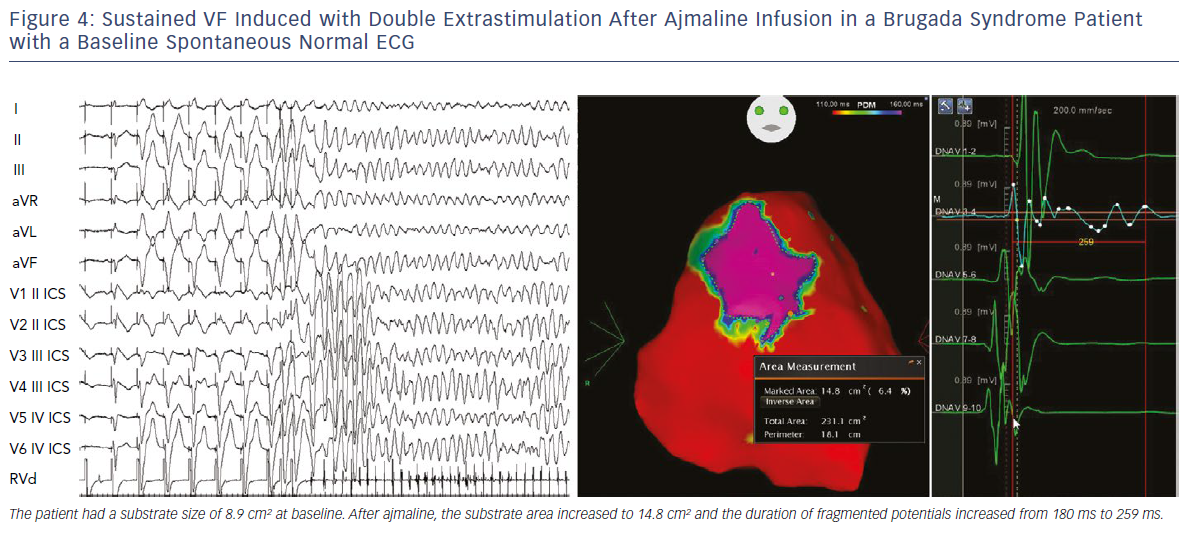
We also demonstrated that, independently from clinical presentation, inducibility by a single or double extrastimuli reflected larger substrates than inducibility by three extrastimuli (Figures 3–5). These original observations support the concept that BrS is a complex disease characterised by large but potentially reversible abnormal substrates representing the primary mechanism of malignant VT/VF. Unlike traditional stable substrates, which are characterised by scar or fibrosis as in post-ischemic VT, the impressive variation in size and shape of the BrS substrate, as exposed by ajmaline, clearly suggests that a component is functional rather than a fixed structural replacement with fibrosis. We cannot exclude the fact that in the natural history of BrS over-exposure to specific triggers can facilitate substrate progression from functional to structural changes, as observed in patients with frequent electrical storms.23 Therefore, in BrS, epicardial ablation of all abnormal potentials areas should be guided by repeated infusion of ajmaline to unmask the entire substrate size in order to eliminate multiple re-entrant circuits leading to rapid unstable ventricular arrhythmias and VF.
It is conceivable that a substrate-based, interventional approach can pave the way to a cure for BrS, potentially removing the need for ICD implantation or chronic quinidine therapy, as suggested by the preliminary results over short-term follow-up reports of >300 patients worldwide.25 However, epicardial ablation may be associated with potential risks and complications due to epicardial access and RF applications. Therefore, this procedure should be performed in highly experienced centres and ajmaline administration should be performed after patients are counselled appropriately for the potential arrhythmogenic implications of drug administration.
Clinical Perspective
- The discovery of a dynamic frequently hidden arrhythmic substrate in Brugada syndrome (BrS) has provided new insights into pathophysiology and management of patients with BrS.
- Ajmaline can prolong the duration and fragmentation of abnormal epicardial electrograms and reveal an arrhythmic substrate extending beyond the right ventricular outflow tract (RVOT).
- Re-induction of coved-type ECG by ajmaline after epicardial ablation is caused by residual abnormal electrograms in the corresponding epicardial RVOT region, while disappearance of coved-type ECG is due to elimination of the remaining epicardial substrate.
- These findings are clinically relevant suggesting that epicardial ablation, as guided by ajmaline infusion, may be considered as a new effective therapeutic option in BrS.