AF is a common arrhythmia in clinical practice, with a prevalence of 2.7–6.1 million that is expected to rise to 5.6–12 million by 2050 in the US alone.1,2 Patients with AF have an increased risk of stroke and mortality and a decreased quality of life.3 In addition, management of AF increases the cost of healthcare.4,5,6 The mechanisms of AF are complex and are associated with electric and structural remodelling.7,8 What comes first: the AF or the atrial tissue damage or myopathy? A ‘chicken or egg’ question.
Over the last decade significant developments in imaging atrial and ventricular tissue using cardiac MRI (CMR) have led to a measurable advancement in AF management. Utility includes localisation and quantification of the extent of cardiac substrate or myopathy, as well as the cardiac chamber shape, size and function.9–11 In this review, we highlight the most recent innovations and advances in the role of CMR in defining the AF substrate and the implications for the management of AF.
Cardiac MRI Acquisition and Processing
Quantification of left atrial (LA) structure and function using CMR has previously been presented.9,12–14 In brief, the MRI scan is performed on either a 1.5 or 3 Tesla scanner using conventional body and spine phased-array coils or specialised cardiac coils. Cardiac magnetic resonance angiography (MRA) is acquired during continuous gadolinium-based agent injection. High-resolution 3D late gadolinium enhancement (LGE) scans of the LA are typically acquired 15–30 minutes after contrast agent injection in the same imaging session. The imaging technique for LGE-MRI is based on respiratory navigated, ECG-gated, gradient echo pulse sequence with fat suppression and inversion recovery preparation. To minimise the effect of LA motion, imaging data are acquired during the diastolic phase of the cardiac cycle prior to atrial kick. Data acquisition is usually limited to 15–20% of the cardiac cycle. Scan time for LGE-MRI of the LA is expected not to exceed 5–12 minutes, depending on patient heart rate and respiratory pattern. The typical scan parameters are a transverse imaging volume with voxel size of 1.25 × 1.25 × 2.5 mm (reconstructed to 0.625 × 0.625 × 1.25 mm) and inversion time of 230–320 ms.13
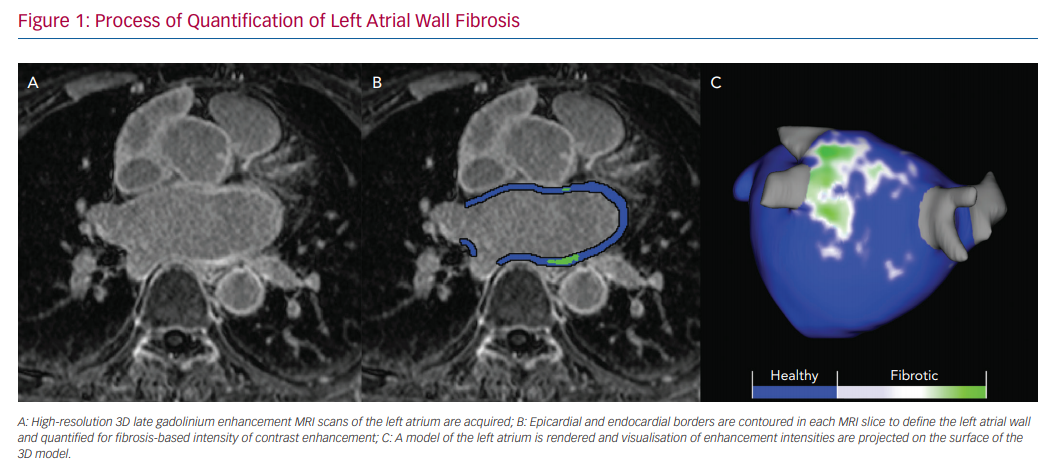
Several tools have been developed to analyse images acquired with CMR. Most of our experience has been with Corview (Marrek), a clinical and research software used to stage atrial myopathy and LA morphology in patients with AF.13,15,16 In summary, the epicardial and endocardial LA boundaries are segmented using a semi-automated fast grow-cut algorithm and then further refined by manual contouring.17 A 3D model of the left atrium is rendered, and atrial tissue changes are quantified by selecting intensity thresholds that correspond with LGE in the LA wall (Figure 1). Intensity thresholds in the range of 2–4 standard deviations from the mean are used to detect enhanced tissue.
Atrial Myopathy and AF
Clinical and experimental studies have demonstrated a correlation between AF and atrial myopathy and vice versa. In histological examinations, the presence of AF is always associated with varying atrial myopathy in both atria.18,19 AF is known to initiate and perpetuate electrical and structural remodelling, which can ultimately lead to maladaptive consequences including myocardial apoptosis and subsequent collagen deposition, known as replacement fibrosis. Subsequently, this pathological substrate has been shown to maintain AF and can lead to other arrhythmias such as atrial tachycardia and sick sinus syndrome. In addition, this atrial myopathic substrate is also identified in patients with structural heart disease and even those without apparent heart disease.20,21 This indicates that structural alterations are already prevalent before the initiation of AF and AF may represent as an arrhythmic manifestation of the atrial myopathy.10,22,23 Therefore, an earlier and better characterisation of the atrial substrate is of clinical and experimental importance.
The location and extent of atrial myopathy can be quantitatively assessed by CMR. The Delayed Enhancement-MRI Determinant of Successful Catheter Ablation of Atrial Fibrillation (DECAAF) study used CMR and classified LA myopathy based on the extent of LA late enhancement as Utah stages: stage I with <10%, stage II with ≥10% to <20%, stage III with ≥20% to <30% and stage IV with ≥30% LGE. Masson’s trichrome staining of human tissue samples showed that regions with interstitial fibrosis were correlated with high gadolinium enhancement.16 In contrast, minimal collagen staining was detected in the region with low gadolinium enhancement.2 Data from electroanatomic mapping during ablation also revealed that the regions of extreme low voltages correlated with enhanced regions on LGE-MRI.24 The benefit of CMR being non-invasive and having low spatial error, allows for insights into atrial myopathy to be appreciated.
Cardiac MRI and Stroke Risk Assessment of AF Patients
AF patients suffer a fivefold higher stroke risk and AF-related stroke is more likely to be fatal and causes more severe functional disabilities.25 Current guidelines suggest the use of CHA2DS2-VASc score for assessment of stroke risk.26,27 However, conflicting data seem to suggest CHA2DS2-VASc performs poorly in estimating stroke risk.28–30 Emerging CMR markers of atrial myopathy have been shown to strongly correlate with embolic stroke risk regardless of heart rhythm, offering a promising alternative to conventional risk assessment tools.
Left Atrial Fibrosis and Stroke Risk
Atrial fibrosis measured on CMR is an element of stroke risk assessment.31 A retrospective analysis of 387 patients with AF demonstrated that those with extensive LA enhancement had nearly four-times the odds of experiencing thromboembolic events.32 When combined with CHAD (excluding stroke itself) risk factors, a markedly improved predictive accuracy was observed, with the C statistic significantly increasing from 0.58 to 0.72. King et al. demonstrated that a severe LA enhancement was associated with an increased risk of major adverse cardiovascular and cerebrovascular events, mainly driven by elevated risk of stroke.33 Furthermore, LA enhancement on CMR was associated with higher incidence of LA spontaneous echo contrast (SEC) and left atrial appendage (LAA) thrombus formation detected during transoesophageal echocardiography testing.34 This can be explained by increased tissue thrombogenicity and impaired atrial contractility as a result of the atrial myopathy.
Left Atrial Function
Quantitative analysis of LA function has been shown to have prognostic value in stroke risk assessment.35–39 Assessed by LA reservoir strain with speckle-tracking, each 1% decrease in LA ejection fraction resulted in a 7% increased risk of having a cardio-embolic stroke.38 The association between CMR-assessed LA reservoir function and a history of stroke or transient ischaemic attack (TIA) has been shown in patients with AF.36 This is consistent with a cross-sectional study from Ciuffo et al., who concluded that LA mechanical dyssynchrony during sinus rhythm was associated with a history of stroke/TIA.39 A sub-analysis of the Multi-Ethnic Study of Atherosclerosis (MESA) study demonstrated that reduced total LA ejection fraction on CMR was associated with ischaemic cerebrovascular events independent of clinical risk factors.40 One can expect that a lower LA reservoir function may increase blood stasis and participate in subsequent thrombus formation. Notably, most of these findings are evaluated in AF patients with sinus rhythm. In patients with persistent and long-standing AF, there is a need for more research to obtain an integrated analysis of LA function and stroke risk.
Left Atrial Morphology
Studies now focus on features of LA shape on MRA and its relationship with stroke risk. Bisbal et al. described the LA sphericity analysing the LA geometry by CMR and claimed a higher LA sphericity was the only factor associated with prior thrombus events with an OR of 1.26 for each 1% increase in LA sphericity.41,42 It stands to reason a more spherical shape has more areas of stagnant flow and may reduce the generation of eddy current and promote the formation of blood stasis and thrombosis. Cates et al. developed a more descriptive and comprehensive shape score for identifying LA shape changes using particle-based modelling (PBM).14,43 They extracted the LA surface contours from MRA, and used the LA endocardial surface correspondence points to calculate the ratio of the maximum anterior to posterior distance to the maximum left to right distance.14 Shape scores are computed by coefficients from the model and LA shape is divided into four classes. From this PBM-based method, a higher LA shape score tends to have a more spherical shape and thus potentially serve as a substrate for stroke development. Clinical studies are needed to relate this score system to stroke risk.
Left Atrial Appendage Morphology
The LAA is responsible for approximately 90% of the thrombus in patients with nonvalvular AF.44,45 Beinart et al. analysed the geometry and dimensions of the LAA derived from CMR of 144 patients.46 A larger LAA neck dimension is associated with a history of TIA or stroke in AF patients. Cates et al. applied PBM to LAA acquired with CMR and compared LAA morphology between patients with and without SEC based on LAA length and orientation parameters.14 Morphologies with longer, thinner LAAs and LAAs that curved anteriorly were more likely to present SEC on transoesophageal echocardiography. The underlying mechanism may be that a longer and more curved LAA structure might be more restrictive of blood flow in the chamber, increasing blood stasis and thus stroke risk.
In summary, we propose that atrial myopathy markers on CMR including LA fibrosis, LA and LAA morphology and LA function could indeed have strong predictive value in stroke risk assessment. These CMR markers may be implemented into risk stratification methods for AF. Large clinical trials focused on validating CMR-based morphometric analyses are warranted to revolutionise stroke prevention strategies and provide more accurate, personalised stroke risk management for high-risk patients with and without AF.
Cardiac MRI and AF Treatment Strategy
Cardiac MRI Helps Define a Treatment Plan
Catheter ablation of AF is emerging as a first-line treatment option to restore sinus rhythm and improve long-term clinical outcomes.47,48 Despite dramatic improvements in techniques over the last 2 decades, the short and long-term success rate of catheter ablation is still modest.49–52 Moreover, the catheter ablation community is still operating under ‘one-size-fits-all’ approach. Not every patient is an ablation candidate – personalised ablation strategies are urgently needed.
Over the last 13 years, a significant amount of data has emerged supporting the use of CMR in defining appropriate candidates for catheter ablation independent of approach and tools used. In the DECAAF study, the pre-ablation extent of LGE was an independent predictor of arrhythmia recurrence.16 A baseline LGE extent of more than 30% was associated with a poor response to the procedure in the first year after ablation.53 In a 5-year follow-up study, every 10% increase in atrial fibrosis pre-procedure accounted for a 45% increased risk of AF recurrence.54 Moreover, patients with minimal LA enhancement experienced better outcomes after ablation.
LA remodelling is associated with change of LA geometry, leading to a greater LA diameter and higher LA volume.55,56 Functional measurements by echocardiography does not allow appreciation of LA shape. Bisbal et al. introduced the concept that LA sphericity measure by CMR was associated with a larger LA diameter and higher risk of AF recurrence.41 LA functional remodelling also serves as a key factor to atrial myopathy. Histologic analysis demonstrated that decreased LA function as measured by CMR strongly correlates with increased fibrofatty myocardial replacement.57 In patients with AF, the lower pre-ablation function was correlated with higher LA enhancement and lower AF ablation procedural success.58
Cardiac MRI During Ablative Treatment of AF
Applying CMR imaging before treating AF also helps define an ablation target. A sub-analysis of the DECAAF study suggested that residual fibrosis, defined as preexisting fibrosis not altered by the ablation procedure, was associated with higher incidence of recurrent atrial arrhythmia.59 This observation was confirmed in another 172-patient study demonstrating that a higher residual fibrosis correlated with poor ablation success rate.60 This evidence suggests that targeting areas of atrial myopathy during an ablation procedure could convert a heterogeneous arrhythmogenic fibrotic tissue to homogeneous scar tissue. This so-called scar homogenisation could lead to improvement in procedural outcome. The on-going DECAAFII trial enrolled more than 800 AF patients and aims to investigate the hypothesis that targeting atrial myopathy during catheter ablation can improve the treatment success rate, as well as clinical outcomes (NCT02529319).
Real-time MRI-guided ablation has shown great potential in improving the catheter ablation procedure. It is useful in visualising and localising both ablation lesions and scar formation in animal models.61,62 Data of real-time MRI-guided electrophysiology in patients are limited.63–66 Nazarian et al. reported the first successful electrophysiological study in two patients.63 A recent pilot study demonstrated that real-time CMR-guided ablation for typical right atrial flutter is safe and highly efficacy.66 Until now, real-time CMR-guided ablation is not yet applied to AF patients. In addition, advanced CMR devices and imaging techniques are essential to broad clinical use.
Role of Cardiac MRI in Patients After Ablative Treatment
Detection of ablation lesion after ablation of AF is a major strength of CMR.67–70 Pulmonary vein (PV) reconnection is a main reason for AF recurrence. In a study by Badger et al., circumferential scarring of all four PVs was only achieved in 6.9% of patients.69 Nevertheless, many patients with at least one non-isolated PV remained in sinus rhythm.59 Poor scar formation transferred from acute electrical isolation is also a key factor. Although electrical isolation was achieved during the AF ablation procedure, only 33.9% of lesions were permanently scarred 3 months later on CMR.67
CMR can quantify and localise the gaps among PVs and ablation-induced scar. An increase of 10% relative gap length increased the likelihood of AF recurrence by 16%.71 This emphasises the potential benefit of targeting CMR-detected gaps as a feasible approach during repeat ablation. Among 102 patients who underwent second procedure, Fochler et al. used a de-channelling ablation procedure involving targeting channels/gaps and superficial ablation lesions as detected by either electroanatomic mapping or post-ablation CMR. They found that after 1 year of follow-up, patients had similar recurrence rates regardless of the de-channelling ablation strategy whether it was guided by electroanatomic mapping or CMR.68 In patients with repeated procedures, aggressive ablative strategies are always recommended. However, high scar burden leads to a reduction of LA function independent of AF recurrence.72,73 Based on the pre-repeat procedure CMR, the operator can save time and effort spent on the electroanatomic mapping, as well as avoid extensive ablation and scar formation.
Moreover, atrial myopathy is a dynamic disease. A recent study marked fibrotic progression ≥21% after catheter ablation as a novel predictor of long-term procedural success. For every 1% increase in new-onset fibrosis, the risk of post-ablation AF recurrence increased by 3%.74 On the other hand, atrial myopathy may continue to exist independent of AF. In patients with lone AF, the subtle atrial dysfunction did not normalise after ablation and this further indicates that atrial myopathy may be a cause of arrhythmia.75 Therefore, it is important to monitor atrial myopathy even in patients without recurrence.
Personalised Approach for AF Management Based on Cardiac MRI
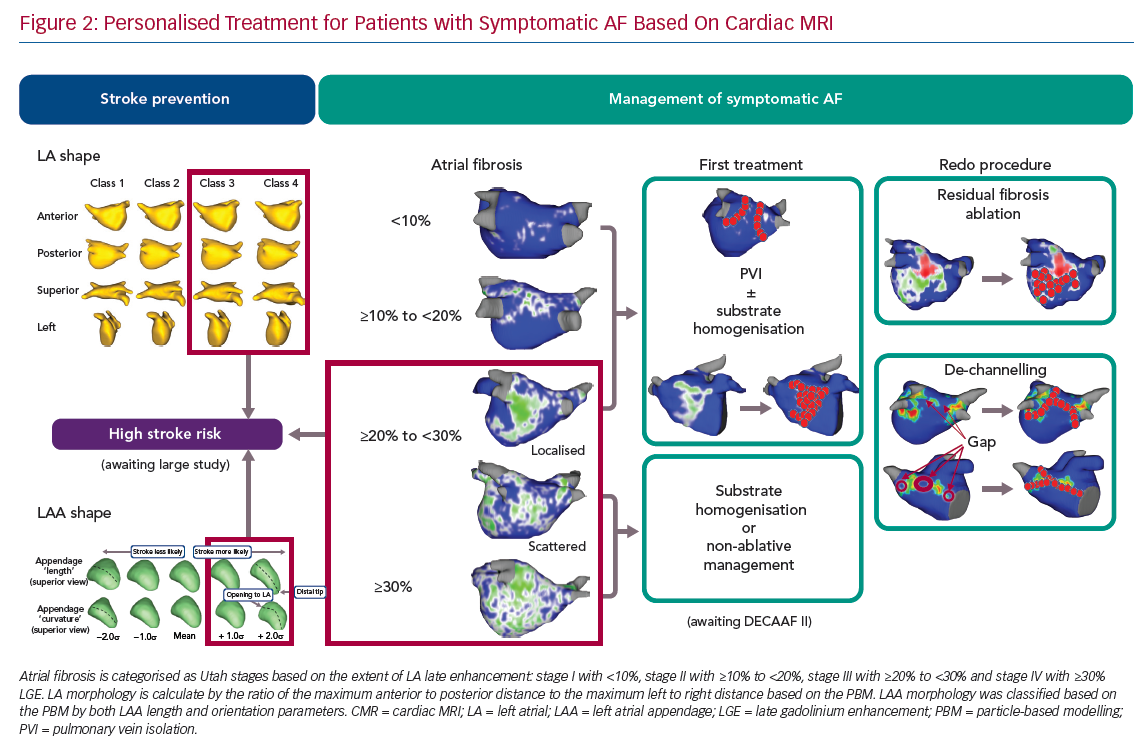
The understanding of AF is changing from a sole rhythm disease to that of an atrial myopathy disease.10,22,23 Recent innovations in imaging techniques help advance the concept of atrial myopathy as a clinically relevant entity. CMR is valuable in characterising the thrombogenic and arrhythmogenic remodelling process associated with atrial myopathy. From this perspective, we have developed a treatment algorithm to individualise AF ablation strategies (Figure 2). Based on the data discussed above, we recommend ablation as a first line therapy for patients with low extent of LA fibrosis (e.g. Utah stage I and Utah stage II). For patients with higher Utah classes (e.g. Utah stage III with diffuse fibrosis and Utah stage IV), a non-invasive approach or fibrosis homogenisation should only be considered. The DECAAF II study will provide more insight into atrial myopathy and guidance on its treatment. Gaps between ablated-scar and progression of atrial myopathy should be considered in cases of arrhythmia recurrence. It needs to be stressed that – regardless of the treatment – monitoring ablation lesion behaviour and progression of atrial myopathy using CMR is necessary.
Atrial myopathy markers detected by CMR also predict the risk of having a cardio-embolic stroke. As such, anticoagulants could be prescribed for patients with extensive atrial myopathy, regardless of the CHA2DS2-VASc score. Moreover, anticoagulation should be continued after ablation even without evidence of recurrent atrial arrhythmia. Large clinical trials are needed to verify this treatment algorithm and establish a more powerful prediction model based on imaging markers to better personalise treatment of AF.
Conclusion
AF and atrial myopathy are two epidemics that often coexist with complex bidirectional interactions. With recent developments in advanced imaging techniques, CMR, in particular, is establishing itself as a powerful tool for assessment of cardiac myopathy and guiding treatment strategies for the AF patient. Further standardisation and large randomised clinical trials are needed to integrate personalised CMR algorithms into definitive guidelines and revealing a new era in the treatment of the atrial disease.
Clinical Perspective
- Current understanding of AF has been enhanced from a sole rhythm disease towards a cardiomyopathy based on arrhythmia substrates.
- CMR is a viable tool for characterising the thrombogenic and arrhythmogenic remodelling process associated with atrial myopathy.
- Applying CMR for AF patients allows for a strategy of an individual and substrate-guided management of AF.