Patients implanted with a pacemaker (PM) or implantable cardioverter defibrillator (ICD) require regular follow-ups to control the proper function of the implanted device. These technical checks have traditionally been performed manually in-office using a dedicated device programmer. In 1971, transtelephonic monitoring was introduced to remotely follow-up basic parameters (such as battery status and thresholds) of PMs. Many modern PMs and ICDs are able to automatically perform technical checks such as battery status, lead impedance, and sensing and pacing thresholds. With the evolution of communication technology, remote device management has become available that allows PMs or ICDs to automatically transmit this information to the physician. Current guidelines stipulate that the patient should be seen in person at least once a year until battery depletion, with remote management being possible after initial post-operative follow-up.1 When alluding to remote device management, a distinction should be made between remote follow-up (which involves scheduled automatic device interrogations), remote monitoring (which involves automatic unscheduled transmission of alert events such as onset of atrial fibrillation) and patient initiated interrogations (which are full device interrogations initiated manually by the patient, e.g. in response to symptoms).2 Remote device management is widely implemented in the US, where it is reimbursed since 2006, and is being increasingly adopted in Europe.3 This article aims to briefly overview the current status of remote device management.
Existing Systems
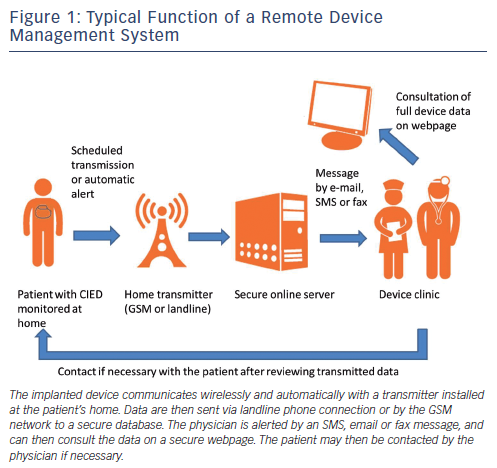
Remote device management for PMs and ICDs was pioneered by Biotronik, and all other major device companies (Boston Scientific, Medtronic, Inc, Sorin and St. Jude Medical) now also offer their own systems. These function in a similar manner, although they do have technical differences. Older implantable devices require a telemetry wand for manual interrogation by the patient, which is an obvious setback. Recent implantable devices have an incorporated antenna that allows wireless automatic data transmission with a unit installed in the patient's home, and that does not require any action by the patient (other than correctly setting up the system). The data are sent via landline phone or the Global System for Mobile communications (GSM™) network to a secure database server. A message is then sent to the physician by email, short message service (SMS) or by fax (depending on the system and its configuration), who may then consult the data via a secure internet access (see Figure 1). None of the existing systems currently allow remote programming of the implanted device, mainly for security reasons (although this would be technically feasible).
Is Remote Follow-up Safe?
It has been shown that <10 % of all scheduled in-office visits are 'actionable' (i.e. that they result in changes in medication or device programming). A study reported that 94 % of scheduled in-office ICD clinic visits may just as well have been performed remotely.4 Remote device follow-up allows to safely reduce the numbers of in-office visits, which is attractive both from the patient's perspective (less travel and waiting time) as well as the healthcare provider's perspective (quicker and more flexible followup). In the Lumos-T Safely RedUceS RouTine Office Device Follow-Up (TRUST) trial,5 1,339 patients with a single- or dual-chamber ICD were randomised to three-monthly in-office versus remote follow-ups (with an in-office follow-up performed after implantation and at 12-months in all patients). There was a 45 % decrease in numbers of in-office visits in the home monitoring group (2.1/year versus 3.8/year, P<0.001), without any increase in adverse events. In this trial using wireless transmitters with a simple and automatic setup process, 91 % of the daily transmissions were successfully transmitted to the device clinic, ensuring that at any stage recently refreshed data were automatically available for review. A similar rate of 93 % of successful automatic transmission of alerts was reported in the first phase of the MOnitoring Resynchronization dEvices and CARdiac patiEnts (MORE-CARE) trial.6
The randomized trial of long-term remote monitoring of pacemaker recipients (COMPAS) trial in patients with dual-chamber PMs found that home monitoring allowed delaying scheduled in-office visits for as much as 18 months after device implantation, without any significant difference in major adverse events compared with patients with routine follow-up.7 Current guidelines stipulate that all patients should be seen in-office at least once a year.1 However, over a third of ICD or cardiac resynchronisation therapy defibrillator (CRT-D) patients would prefer to be seen in-office at intervals of 18-months or longer.8 It may be that one day, selected low-risk PM patients may be followed up remotely (especially when using devices with remote monitoring, see below) for the entire device lifetime.
Does Remote Monitoring Improve Patient Outcome?
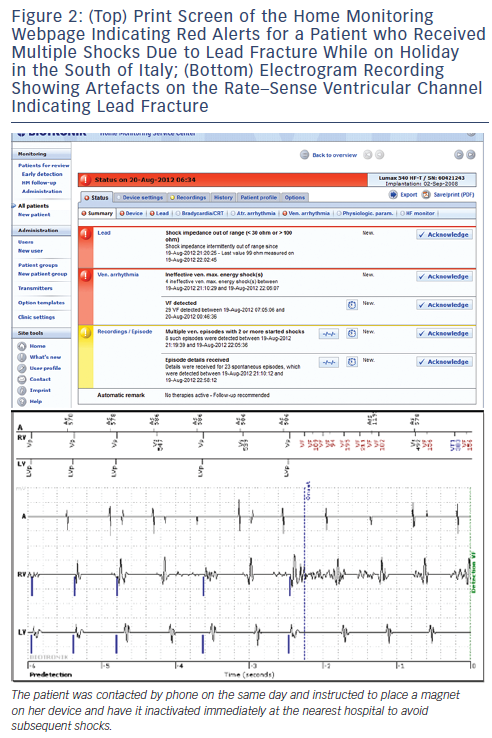
Remote monitoring has been shown to dramatically reduce the time to detection of events such as arrhythmias and technical issues.5-7,9 Remote monitoring of cardiac arrhythmias, heart failure status (by parameters such as heart rate, daily activity, lung fluid, etc.) as well as device integrity (see Figure 2), has the potential to improve patient outcome. Data analysed as secondary endpoints from several trials are encouraging. In the Clinical Evaluation of Remote Notification to Reduce Time to Clinical Decision (CONNECT) trial9 randomising 1,997 patients implanted with a dual-chamber or biventricular ICDs to remote monitoring versus in-office visits, there was an 18 % reduction (P=0.002) in the length of stay for cardiovascular hospitalisation in the remote monitoring arm. This led to an estimated cost-saving of US$1,793 (95 % confidence interval: US$1,644-1,940) per hospitalisation. In the COMPAS trial7 patients on remote monitoring had a significantly reduced risk of hospitalisation for atrial arrhythmias or for stroke (P<0.05). Incidence of inappropriate shock was also reduced by remote monitoring from 10.4 to 5.0 % (P=0.03) in a sub-analysis of the Effectiveness and Cost of ICD Follow-Up Schedule with Telecardiology (ECOST) trial.10 In the ALTITUDE registry, among 10,272 matched subjects implanted with an ICD or a CRT-D device, patients who were on remote monitoring had an approximately 50 % relative reduction of total mortality compared with those on standard care.11 In the Evolution of Management Strategies of Heart Failure Patients With Implantable Defibrillators (EVOLVO) study,12 patients randomised to remote monitoring had a 35 % (P=0.005) reduction in emergency visits for arrhythmias, heart failure and device-related events compared with a control group with audible alerts (which probably increased the number of emergency visits in this study). These data are encouraging, but need to be confirmed by adequately powered randomised trials, several of which are currently underway.13,14
Which Patient Should Receive Remote Device Management?
There are currently no guidelines on which patient should be followed by remote device management. Some centres include all patients implanted with a device equipped with wireless technology, but current practice is to choose patients on a case-by-case basis. Travel distance and patient mobility should be considered for remote follow-up. Elderly, debilitated patients benefit from remote device management, with participation of the patient's family or caregivers if manual transmissions are necessary.15 Patients who are professionally active or who spend considerable time abroad are also good candidates for remote follow-up. As it concerns remote monitoring, the sickest patients (e.g. those most likely to present arrhythmias, heart failure, or who are pacemaker-dependent) are those who may benefit the most. Likewise, devices that are most prone to technical issues (e.g. cardiac resynchronisation therapy, leads under recall, batteries nearing elective replacement, etc.) are most likely to generate alerts that are of clinical relevance.
Implications for Workflow
Whereas remote follow-up is a convenience for both patients8,16 and caregivers,17 remote monitoring adds work burden to the device clinic. Phone contact with the patients in response to the alerts can be time-consuming and may increase patient anxiety. Technical troubleshooting, reviewing of alert messages and patient contact in response to these alerts may require considerable time and effort. Most centres have a device nurse who periodically logs on to the secure servers to perform remote follow-up and to deal with the alert messages. The nurse may perform triage of the alerts (local protocols on how to deal with the different alerts are useful in this respect), and thus filter the data that require attention by the physician. In a study with 117 device patients on a remote monitoring system, a nurse spent 59 minutes/week screening the messages, with 12 minutes/week by the cardiologist to deal with issues that required specific attention.17 Automated algorithms based upon integrated diagnostics (e.g. combining data for lung fluid overload with other parameters18) may also help triage alerts, but these need to be validated in clinical trials.
Patients often express a desire to know about the results of their device check, whether in-person or remote. Results of an automatic remote follow-up may be communicated by sending a letter, but some systems allow automatic feedback to the patient (e.g. a text or voice message, St. Jude Medical Merlin.net®) or via a message on the home monitor screen (Boston Scientific LATITUDE®). As current and future generations of patients are likely to increase their use of SMS and email, these may be increasingly employed in the future to provide a rapid, efficient and cheap means to communicate results.
It is accepted that remote monitoring is usually only provided during office hours, and patients should be made aware that it does not replace emergency healthcare.2 Many centres require that the patient signs an informed consent form that explains these points.
Economic Aspects
A review on the economic implications of remote device management for patients, manufacturers, caregivers and payers has recently been published.19 A Markov model using data from published trials reports that remote device management generates negligible costs after seven years of use (and is increasingly cost-saving after 10 years) despite the initial cost of the home transmitter of £1,334.20 This is mainly due to prolongation of battery life (resulting from a reduction in shocks). In the EVOLVO study, patients on remote monitoring had a reduction of emergency room visits compared with those with audible alerts), leading to cost-savings of 888 euros/patient.21
Trials are currently underway in Europe that will be useful to make more accurate evaluations of the financial impact of this technology. Remote follow-up of PMs and ICDs has been reimbursed in the US since 2006, in Germany since 2008 and also in a few other countries, but still needs to be addressed across most of Europe.2,22
Future Perspectives
There is room for improvement for some systems to avoid recurrent technical issues (mainly related to data transmission) and ease of utilisation (e.g. avoid requiring in-office visits to reset alerts, full online configuration of alert settings, more possibilities of communicating with the patient via the transmitter, etc.). Energy consumption by the system should be minimised to avoid premature battery drain. Daily transmissions by the Home Monitoring® system of Biotronik for instance consume the equivalent of only a single maximal energy shock over the lifetime of the device according to the manufacturer. The Medtronic system consumes approximately 1-2 days of device longevity for each transmission,23 which are therefore usually performed at intervals ofseveral weeks or months. Prolonging time-to-box replacement by monitoring battery voltage and reducing numbers of shocks with remote monitoring should, however, offset the excess consumption. Data transmission by the GSM network is usually preferred to landline transmission, and most systems have implemented this, at least as an option. Another important technical aspect is that the implanted device should be able to perform all routine measurements (e.g. pacing thresholds of all leads, especially of the left ventricular lead) to be able to replace in-office visits (not all devices are currently able to do so).
Direct importation of interrogated data into electronic medical records (EMR) would be a great asset to staff performing device follow-up. Even though most systems are Health Level Seven (HL-7) compatible, few allow direct importation into EMR. A common platform that allows importation of data from most major device companies and exportation to the hospital EMR is commercially available (MediConnect® from Fleischhacker, Germany). Another aspect is the wealth of data available on remote monitoring databases for conducting clinical research.11 The databases are also useful for tracking and reporting product performance.
Conclusions
Remote management of PMs and ICDs is preferred to in-office follow-up by many patients and physicians. It will be increasingly adopted to deal with the growing number of device patients, and is also likely to improve outcome. However, the issue of reimbursement needs to be properly addressed by healthcare authorities of most European countries, with economic models tailored to local requirements that will allow this technology to be viable in the long term.