Catheter ablation of AF has become an established therapy and may have the potential to cure this most commonly encountered sustained arrhythmia. Previous studies have demonstrated that pulmonary veins (PVs) are a major source of the ectopic beats that initiate AF. PV isolation in patients with symptomatic paroxysmal AF refractory to antiarrhythmic drugs is effective; however, it is difficult to eliminate all instances of AF.1–3 If ectopic foci consistently come from a non-PV area and a pattern of spontaneous onset of AF is onset confirmed, the earliest ectopic site is defined as the non-PV trigger initiating AF.2,4–7 Ectopy originating from non-PV areas can initiate AF and can cause it to recur after PV isolation.4–31 Non-PV ablation after multiple AF ablation procedures decreases the risk of recurrence and increases the cure rate.10,19–21,23,25,28,29 Although several ablation strategies have been developed, the outcomes of ablation are not improved unless substrate modification targets AF triggers.30 Taking all of these considerations into account, non-PV ectopy plays important role in both AF initiation and recurrence.2,4–7,20,29,30,32–34
Mapping studies of non-PV foci have revealed that triggers are often found in anatomically predictable regions, such as the left atrial wall, thoracic veins and crista terminalis, and can be sustained or non-sustained triggers of AF. These areas can be mapped by specific multielectrode catheters positioned in key regions and ablated after the AF is induced and localised, or they can be ablated empirically without the induction of ectopy.1–34 This review focuses on catheter ablation of AF initiated by non-PV triggers, summarising the electrophysiological characteristics, mapping and ablation strategies, their safety and efficacy.
Electrophysiological Features of AF Originating from Non-pulmonary Vein Areas
Incidence of Initiators
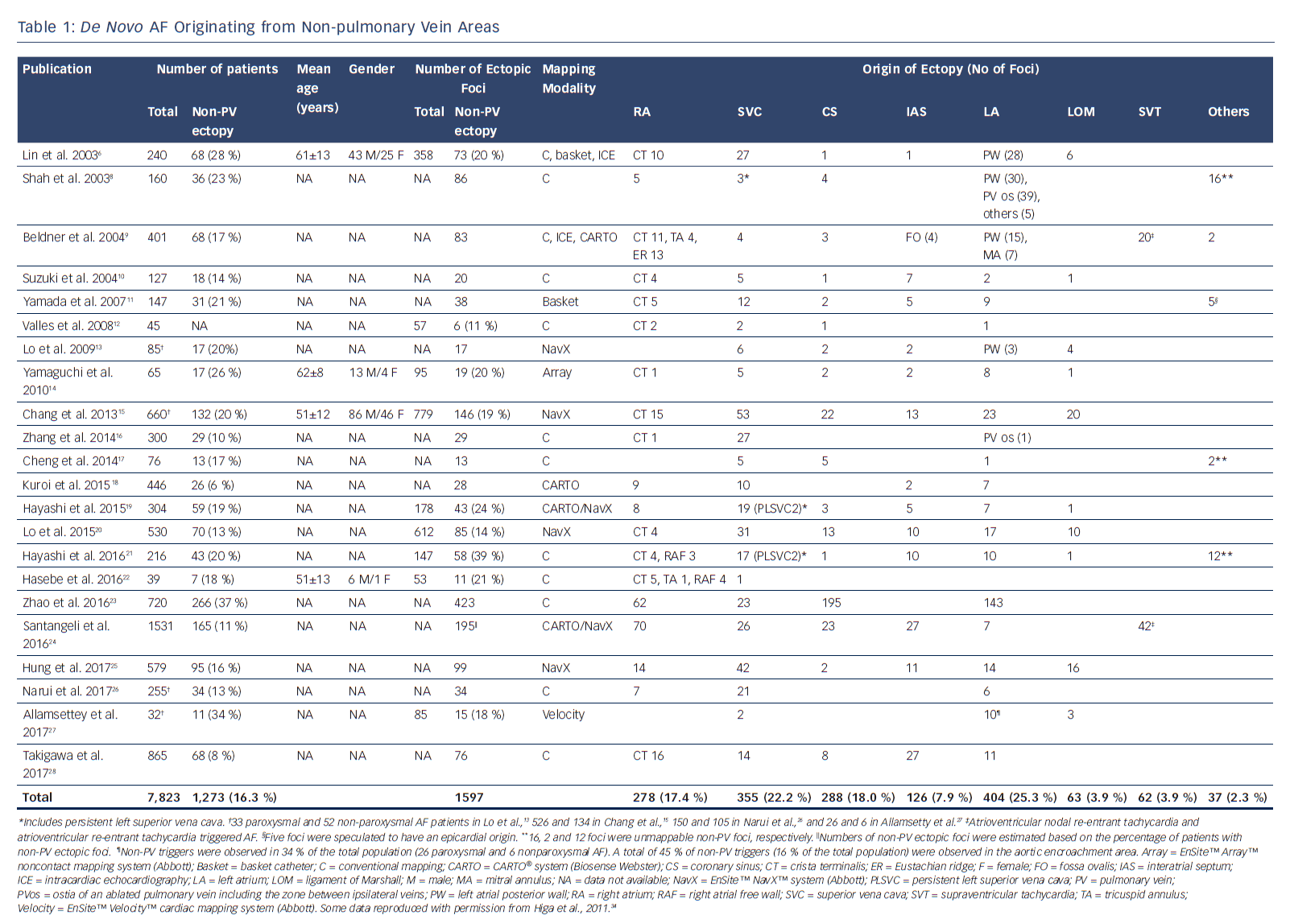
Several important concepts have been proposed regarding the role of non-PV ectopy in initiating AF.2,4–7 AF is initiated by non-PV disturbance of the cardiac rhythm in up to 39 % of cases.3,8–10,32–38 The left atrium (LA) (25.3 %), superior vena cava (SVC) (22.2 %), coronary sinus (CS) (18.0 %), right atrium (RA) including the crista terminalis (17.4 %), interatrial septum (7.9 %), and ligament of Marshall (LOM) (3.9 %) are the areas in which non-PV triggers of de novo AF are most commonly found (Table 1), whereas the SVC, interatrial septum and LA are the most common non-PV trigger sites in recurrent AF (Table 2).6–30 Furthermore, there is a higher incidence of non-PV triggers initiating AF in females and in patients with an enlarged LA.39
Pathophysiology
Histological analysis of the embryonic sinus venosus has identified areas capable of spontaneous depolarisation at the junctions between different embryonic tissues, such as the RA–SVC junction, crista terminalis and CS ostium.40–42 The SVC is a major origin of non-PV triggers of AF.5,8,32–34,43–47 Heterogeneity of the SVC sleeve and arrhythmogenicity of cardiomyocytes isolated from the SVC have been reported.41,42 An excitation from the SVC can conduct to the RA through the myocardial extensions of the SVC sleeve.48–50 Diseased human atria are hypopolarised in comparison to normal atria, which may account for the abnormal automaticity and/or activity originating from the LA wall.51–53 The crista terminalis, which is an area exhibiting abnormal automaticity, anisotropy and slow conduction, may serve as an arrhythmogenic substrate for AF initiation and perpetuation.54,55 Catecholamine-sensitive ectopy arising from the crista terminalis exhibits high-frequency depolarisations with fibrillatory conduction.6 The LOM is an embryological remnant of the left SVC and contains arrhythmogenic myocardial fibres with sympathetic innervation. Several reports have demonstrated the existence of catecholamine-sensitive tissue within the LOM that has abnormal automaticity, and which could be a potential source of AF initiation.6,56–58
The musculature within the CS also has arrhythmogenic activity, with spontaneous depolarisations induced by catecholamine loading.59,60 Abnormal dilatation due to an unroofed CS can be an arrhythmogenic focus of AF initiation.61 A recent study found that 45 % of non-PV triggers of AF were in the area of aortic encroachment, which equates to 16 % of the total population with AF. There is also an arrhythmogenic substrate exhibiting low voltage and fractionated electrograms with a prolonged duration in the anterior part of the LA at the site of aortic encroachment.27
Diagnosis
Provoking Ectopy
To successfully provoke ectopy with AF initiation, antiarrhythmic drugs should be discontinued for a period of at least five half-lives before the patient undergoes electrophysiological study. Spontaneous initiation of ectopic beats preceding AF should be observed at baseline or after isoproterenol loading.32–34 In the case of deep sedation or general anaesthesia, it is necessary to give the patient a high dose of isoproterenol to induce ectopy with AF initiation. Adenosine or adenosine triphosphate can also be used, especially in young patients with vagal AF and with a family history of AF.18
If ectopy does not occur, short-burst atrial pacing can be delivered with intermittent pauses or, failing that, atrial burst pacing to induce sustained AF. Careful monitoring for spontaneous reinitiation of AF is required after internal or external cardioversion. The induction of spontaneous AF initiation should be attempted at least twice to confirm the location of ectopy, the initiation pattern of spontaneous AF, and the earliest activation site (the AF initiator).2,4–7,32–34
Mapping
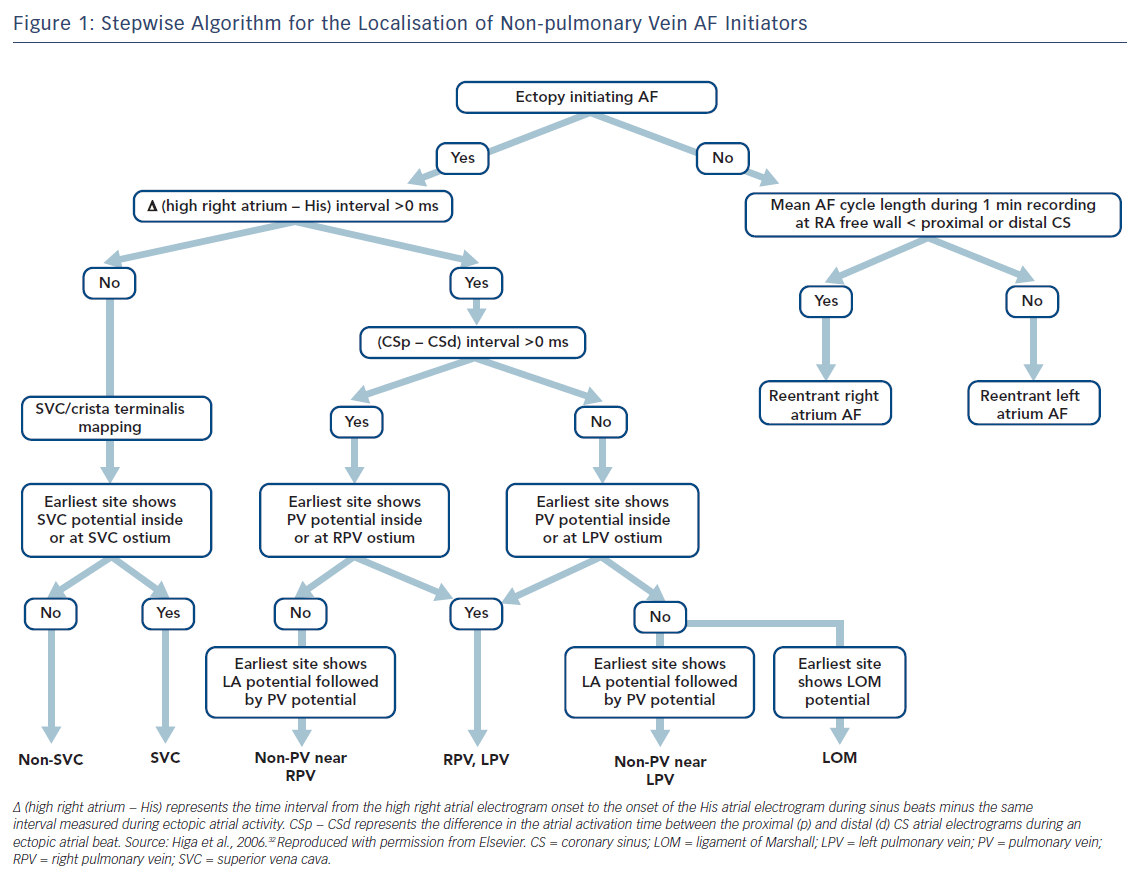
Localisation of AF triggers is important for the catheter ablation of AF. If it is suspected that the trigger is based in the LA, a decapolar catheter should be inserted into the CS via the internal jugular vein and a circular mapping catheter placed in the LA using a transseptal approach. If the initiator is likely to be in the RA, a duodecapolar catheter can be placed from the crista terminalis to the distal SVC for the simultaneous mapping of the right PVs, crista terminalis and SVC. Endocardial activation timing from the high RA, His bundle and distal/proximal portion of the CS can be used to predict non-PV ectopy (Figure 1).2,4–7,32–34 It is 100 % accurate in discriminating ectopy from the SVC or upper portion of the crista terminalis from PV ectopy.32–34,62 The interatrial septum should be the suspected initiator in cases with a monophasic positive narrow P wave in lead V1 or a relatively short activation time (≤15 ms) preceding P wave onset during ectopy. Simultaneous mapping of the right- and left-sided interatrial septum should be performed to avoid any misdiagnosis.32–34,63,64
AF Initiators with Right Atrial Origin
Careful observation of P wave morphology is useful for predicting the approximate location of AF ectopy.32–34,65 A negative P wave or the presence of a negative component in V1 is predictive of an RA origin of AF initiation. Ectopy originating from the SVC or upper portion of the crista terminalis exhibits upright P waves in the inferior leads; ectopy from the CS ostium produces negative P wave polarity in the inferior leads; and ectopy from the middle portion of the crista terminalis results in biphasic P waves. Negative P waves with a long duration in V1 may be associated with RA free-wall ectopy, including the tricuspid annulus.
If RA AF ectopy is suspected, the use of a duo-decapolar catheter is useful for mapping along the crista terminalis to the SVC.2,4–7,32–34 Bipolar signals from the proximal portion of the SVC usually exhibit a blunted atrial signal followed by a discrete sharp SVC signal during sinus rhythm.32–34 The activation sequence of these double potentials is reversed during SVC ectopy. Bipolar signals from the distal part of the SVC usually exhibit double potentials: the first component represents a SVC near-field sharp potential; and the second component, a right superior PV far-field blunted signal. During SVC ectopy, the activation sequence of these double potentials remains unchanged. The activation sequence is reversed during right PV ectopy.
Intracardiac recordings along the crista terminalis also exhibit double potentials during sinus rhythm, with a high-to-low activation sequence.32–34 During crista terminalis ectopy, the atrial activation sequence of the double potentials is reversed. Noncontact mapping using an EnSite™ Array™ (Abbott) can accurately localise the ectopic foci with discrete depolarisations and clarify crista terminalis gap conduction-related small radius re-entry.32–34,66
AF Initiators with Left Atrial Origin
The time interval between the atrial activation of the decapolar catheter in the proximal CS and that in the distal CS is useful for predicting ectopic foci located near the right (>0 ms) or left PV antrum (<0 ms) (Figure 1).32–34,62 During sinus rhythm, the fusion potentials of a blunt signal and a rapid, deflecting sharp signal can be observed in the areas between the LA posterior wall and the PV antrum. The fusion potential consists of atrial and PV signals and can be found at the earliest activation site during LA posterior or PV antral ectopy. An alternating pattern of atrial and PV potentials can also be seen during ectopy.6,32–34
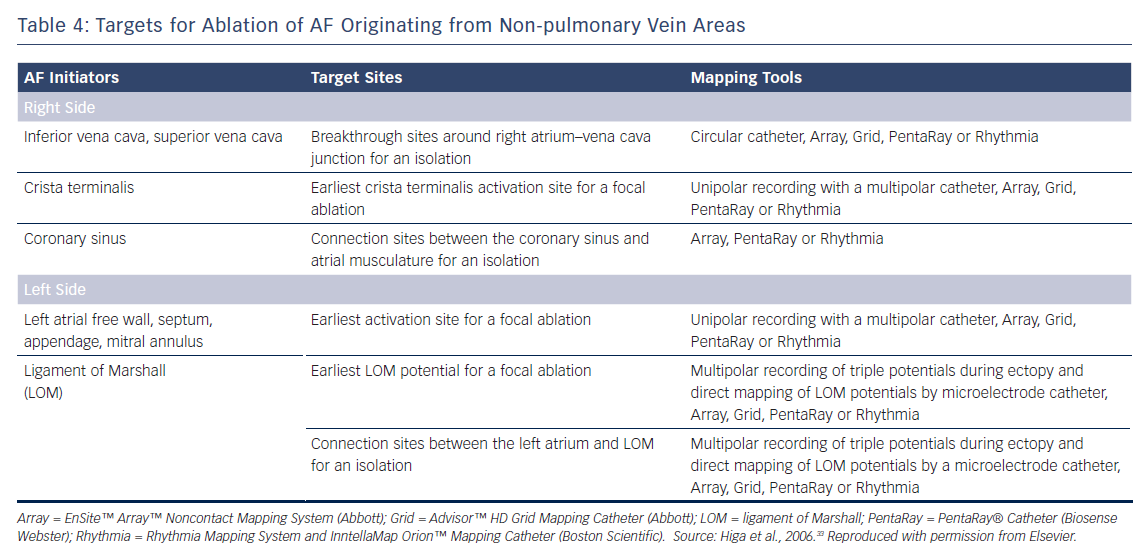
The Marshall ligament has multiple electrical connections to the musculature of the CS, LA posterior free wall and left PV; therefore, it is essential to differentiate a Marshall potential from a left PV or LA posterior free wall potential. A differential pacing method and/or direct recording of the LOM potential by a microelectrode catheter cannulated into the vein of Marshall can distinguish a PV potential from a Marshall potential (Tables 3 and 4).7,32–34,56,67,68
According to expert consensus statements, complex fractionated atrial electrogram (CFAE)-targeted ablation after PV isolation is feasible for substrate modification.69 Interestingly, our laboratory reported a close anatomical relationship between the distribution of CFAEs and non-PV AF initiators. All of the non-PV AF initiators were associated with continuous CFAE sites.70,71 Recently, the efficacy of a novel self-reference mapping technique using a PentaRay® catheter (Biosense Webster) to localise non-PV triggers originating from the LA has been reported.72
Limitations of Mapping
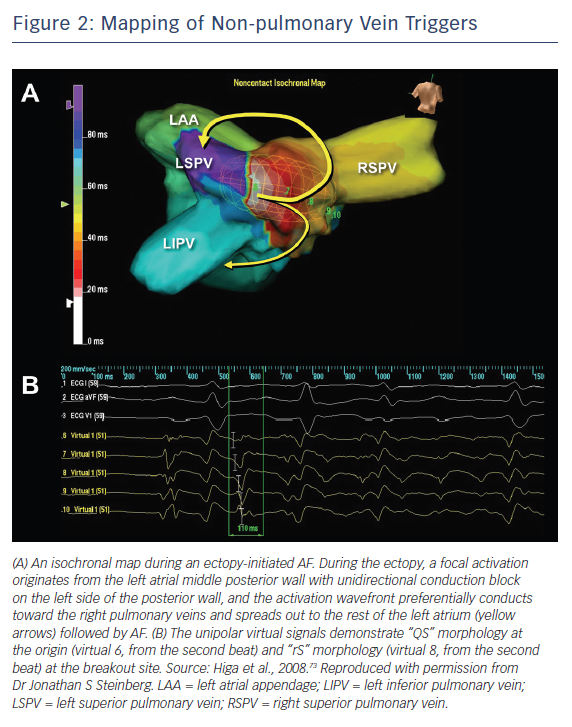
AF ablation can be a challenging and sometimes cumbersome task in cases of unmappable infrequent beats originating from uncommon areas. Activation mapping using fixed multipolar catheters and point-by-point mapping are not efficient for the identification of target ectopies in such cases. Single-beat analysis by noncontact mapping using the Array™ system (Figure 2) or non-invasive body-surface mapping using the CardioInsight™ Mapping Vest system (Medtronic) can be useful tools in these situations.73
Ablation
The earliest bipolar electrogram site with unipolar QS pattern recorded from the origin is the ablation target for non-PV ectopy.2,4–7,32–34 Ablation of an ectopic focus and/or electrical isolation of an arrhythmogenic thoracic vein can be achieved with the application radiofrequency energy for around 30 seconds with a non-irrigated tip at 50–55 °C or with an irrigated tip at <25–30 W.2,4–6,32–34 A contact force catheter can be used to create durable transmural lesions. This method has a lower arrhythmia recurrence and a lower incidence of atrial tachyarrhythmias resulting from incomplete ablation due to proarrhythmic lesion gaps. However, caution should be taken to avoid the application of excessive energy and/or contact pressure at the posterior LA wall close to the oesophagus.
Balloon-based cryoablation of the PV antral area, LA posterior wall and persistent left SVC can also be used to isolate arrhythmogenic myocardium. Complications can be minimised by using a cryocatheter at −30 °C to ice map the area near the atrioventricular node following the cryofreezing (−80 °C) of a para-Hisian or CS ostium trigger. Ice mapping can also be used during ablation near the sinus node when targeting SVC or the upper portion of the crista terminalis. Repeated AF induction protocols should be performed after ablation to assess the non-inducibility of the ablated ectopy.2 4–7,32–34
Vena Cava Triggers
Electrical disconnection between the arrhythmogenic SVC and RA at the level of the RA–SVC junction is the preferred approach for minimising AF recurrence and SVC stenosis in patients with SVC triggers.32–34 The aim is to establish bidirectional (entrance and exit) conduction block between the RA and SVC.5,32–34,47 Circular and basket catheters, and 3D mapping systems including the CARTO® (Biosense Webster), EnSite™ NavX™ Velocity (Abbott), EnSite™ Array™ noncontact mapping system, and Rhythmia HDx™ mapping system with IntellaMap Orion™ catheter (Boston Scientific) can guide SVC isolation.6,32–34,44–46,74–77
Persistent left SVC is also a well-recognised trigger site for AF.19,21,78–85 The connecting musculature means that multiple electrical signals are conducted to the posterolateral LA and middle portion of the CS from the left SVC trigger site. Complete left SVC isolation may be challenging, as it is close to the oesophagus and left phrenic nerve. There have been rare reports of inferior vena cava triggers.86,87 In these cases, the IVC triggers were successfully eliminated with a focal/isolation strategy.
Interatrial Septal Triggers
Focal ablation of the earliest activation site preceding the onset of AF should be performed until a complete elimination of the ectopy is achieved in patients with interatrial septal triggers. Near-simultaneous atrial activation of the multielectrode catheters located in the high RA, His bundle region and CS ostium can be observed in such cases. Simultaneous mapping of the right and left atrial septum is crucial to successfully locate and ablate this trigger.
Crista Terminalis Triggers
Focal ablation of the earliest activation site in the crista terminalis during ectopy preceding AF onset should be performed until complete elimination of the ectopy initiating AF or >50 % reduction in the amplitude of the initial local electrogram at the ablation site.6,32–34 A region with transverse gap conduction in the crista terminalis can be an arrhythmogenic source of re-entry and also ectopy initiating AF. Linear ablation of the transverse gap should address both of these problems.32–34,66 Intracardiac echocardiography can provide real-time monitoring of the anatomical relationship between the crista terminalis and the catheter position during the procedure.
Coronary Sinus Triggers
For patients with a CS trigger, electrical isolation of the arrhythmogenic CS musculature from the atrium by endocardial and/or epicardial ablation under the guidance of a 3D mapping system is preferable.32–34,71 The aim is to eliminate (entrance block) and/or dissociate (exit block) the CS potential.88–91 Care must be taken if an inappropriate impedance rise occurs during CS ablation, and the application of radiofrequency energy should be stopped immediately to prevent any steam pops.
Marshall Ligament Triggers
For patients with Marshall ligament triggers, the earliest site with a LOM potential preceding the onset of AF is targeted using an endocardial and/or epicardial approach.32–34,92 The isolation of both the LOM and left PVs from the LA can be monitored with simultaneous mapping of the LOM and left PV ostia, maximising the chance of a successful procedure.32–34,56,67,93 Ethanol infusion into an arrhythmogenic vein of Marshall through angioplasty guidewire and balloon catheter in addition to PV isolation has recently been reported to have beneficial outcomes.94,95
Left Atrial Triggers
For ectopy from the LA posterior wall, focal ablation of the earliest activation site should be performed (Figure 2). If unsuccessful, a box-shaped linear ablation needs to be added around the ectopy.6,32–34 Box isolation of the LA posterior wall in combination with PV isolation may be a therapeutic option in cases refractory to extensive focal ablation. The endpoint is complete elimination of the ectopy initiating AF, >50 % reduction in the electrogram amplitude of the ectopic focus, or isolation of the posterior LA wall.32–34,73
The left atrial appendage (LAA) has been reported to be a trigger of AF.96 Due to its large structure, triggers may arise from the LAA ostium, body or tip. Simultaneous mapping of the left superior PV and LAA can differentiate between a near-field sharp and a far-field blunt signals and identify true LAA triggers. Focal ablation can be applied to avoid LAA isolation. LAA isolation is only indicated when the patient can tolerate long-standing anticoagulation or a LAA occlusion device is indicated.
Efficacy and Safety of Catheter Ablation
Ablation Outcomes
A relatively high success rate has been demonstrated following the ablation of RA triggers of AF.6 There is a comparatively higher recurrence rate following the ablation of LA triggers. The average success, recurrence and complication rates are 99.3 %, 18.5 %, and 1.9 %, respectively, for AF originating from the vena cava.8,43–45,74,78–87,97–111 These rates are 78.0 %, 16.7 %, and 2.4 %, respectively, for AF originating from the Marshall ligament.6,56,67,68,94,112,113
A higher incidence of recurrent AF and non-PV AF sources has been reported in patients with metabolic syndrome and obstructive sleep apnoea.114 Patients who have a greater extent of left atrial delayed enhancement on MRI have a higher recurrence rate after PV isolation, suggesting the existence of AF triggers in non-PV areas.115
Managing Complications
Overall complication rates are now relatively low as a result of vast improvements in our understanding of the nature and ablation of non-PV ectopy AF triggers. Injury to the sinus node, atrioventricular node, and phrenic nerve, thoracic vein stenosis, peri-oesophageal damage, gastric hypomotility, and pyloric spasms can all be caused by a non-PV trigger ablation.116–124
Complications can be minimised by using a titrated and minimum power setting, short duration of radiofrequency energy, and by monitoring for any sinus rate accelerations, PR or RR interval prolongations and for oesophageal temperature rises. An upstream pacing technique to monitor the phrenic nerve and/or compound muscle action potential can minimise phrenic nerve injury. To reduce the risk of atrio-oesophageal fistula formation, which carries a 60–75 % chance of mortality, surgeons should avoid extensive high-power ablation on the LA posterior and CS walls.119,121,122,125
The use of several luminal oesophageal temperature monitoring systems – SensiTherm™ (Abbott) and CIRCA S-Cath™ (CIRCA Scientific) – and protection systems, including oesophageal warming/cooling devices and deviators to avoid thermal injury, has recently been reported.126–130 Massive air emboli and newly developed thrombi that occur during the ablation procedure can be aspirated.131
Conclusion
Evidence suggests that inducing the non-PV ectopic trigger responsible for initiating AF both before and after PV isolation is an indispensable step in both initial and repeat ablation/isolation procedures. Advances in mapping and alternative energy modalities with 3D navigation are likely to play an important role in the ablation of non-PV ectopy. Together, these advances and the systematic identification of the trigger foci and their successful elimination will improve overall AF ablation outcomes.
Clinical Perspective
- The mechanisms of paroxysmal AF originating from non-pulmonary vein areas are automaticity, triggered activity, and microreentry.
- The diagnosis is made on the basis of a spontaneous onset of the ectopic beats initiating AF during baseline or after provocative manoeuvres.
- The earliest activation sites are the targets for focal ablation.
- The myocardial sleeve surrounding the ostium of the vena cava is the target for isolation.
- Success rates are >99 % for the vena cava and 78 % for the ligament of Marshall.