The His-Purkinje system (HPS) is a complex network of specialised conduction tissue. Jan Purkinje was the first to describe the fibres in 1845, followed by Wilhelm His Jr who made the observation in 1893 that these specialised muscle fibres connected the atria and ventricles and thus severing the ‘His bundle’ resulted in atrioventricular dissociation.1–3 However, it was not until 1906 that Sunao Tawara discovered the right and left bundle branches and accurately determined their role in conduction of excitatory impulses across the ventricular myocardium.4
The left trifascicular system originating from the left bundle was first described in Tawara’s macroscopic delineation of the left ventricular conduction system.4 He described a middle or septal fascicle running in between the commonly recognised left anterior fascicle (LAF) and left posterior fascicle (LPF).5 Demoulin and Kulbertus further refined the anatomical variation of the left-sided trifascicular conduction system by reconstructing transverse histological sections from 20 human hearts. These studies revealed that the left septal fascicle (LSF) emerges in four distinct patterns:
- direct extension from the left bundle;
- extension from the LAF;
- extension from the LPF; and
- contribution from LAF and LPF.6
The functional nature of this septal fascicle has been demonstrated through endocardial activation mapping showing three distinct, simultaneous activation sites.7,8 In addition to the two activation sites represented by LAF and LPF breakout, a third area is activated along the basal third of the mid-left ventricular septum and represents the endocardial breakout from the septal fascicle.
Further branching of the conduction system from the LAF, LPF and LSF forms a dense, complex network of Purkinje fibres that result from both bifurcation as well as merging of two separate fibres. This network is described as a ‘fractal pattern’ rather than a ‘hierarchical tree structure’.9 This is clearly evident from Tawara’s original depiction which showed the complex network of branching articulations and reconnections, resulting in an interconnected web of conduction fibres.5 All of these key anatomical descriptions are critical to understanding the reentrant substrate in the various forms of fascicular-based ventricular tachycardias.
Ventricular arrhythmias dependent on the specialised conduction tissue of the HPS occur in both structurally normal and abnormal hearts. Studies show fascicular-dependent ventricular tachycardias account for about 15% of idiopathic ventricular tachycardias from the left ventricle in structurally normal hearts.10–12 The incidence of ventricular tachycardia (VT) originating from the HPS in structurally abnormal hearts is more difficult to quantify. In one study, about 11% of monomorphic VT post MI were dependent on the fascicular system, while 37% of all inducible sustained monomorphic VT in patients with idiopathic dilated cardiomyopathy were due to bundle branch reentrant tachycardia (BBRVT).13,14
In this review, we discuss the development of the conduction system and the abnormal physiology underpinning the mechanism of reentrant arrhythmias from the HPS. We also review the clinical manifestations and successful management strategies for these arrhythmias.
Anatomy of the Purkinje Fibres and Purkinje Cells
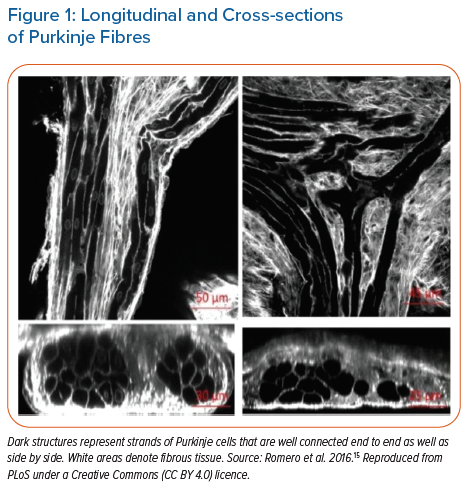
In the 1970s, the isolated Purkinje fibre (PF) was used to study cardiac electrophysiology because it was easy to visualise and isolate it from the heart to be studied in vitro. Free running Purkinje strands appear to have unique end-to-end connections in the longitudinal direction.15 This anatomy is important to note when discussing bundle branch physiology. In adult rabbits, some Purkinje cells (PCs) within a strand have end-to-end connections but also make contact transversally. A bundle of longitudinally oriented cell strands is also tightly surrounded by a connective tissue sheath.15 At the single cell level, a PC has defined gap junctions with a few (1–2 per cell) side-to-side connections of connexin (Cx) 40 and Cx43.16 In some cases, PF bundles are separated by collagen within a larger bundle.15 PCs within these bundles travel longitudinally, but others connect transversely. Thus, internal bundles within a sheathed strand can communicate using a physical network. This microanatomical arrangement is consistent with a set of separated strands running parallel to each other after arising from different sources.
Further, a 3D confocal analysis of PF networks shows that there are two types of Purkinje-ventricular connections.15 One is called a 2D interface and the other is a funnel interface. At a micro level, the tissues of a Purkinje strand could give rise to a reentrant rhythm if there were one or more areas of slowed conduction, such as reduced excitability. On the macro scale, conduction in such Purkinje strands has been observed by others and discussed by Professor Cranefield.17–19 Several reports have described an ‘asynchrony of conduction within strands of Purkinje fibres’. This was thought to be due to longitudinal dissociation of the conducting impulse.20,21 Some arrhythmogenic consequences of longitudinal dissociation during premature stimulation protocols were shown by Myerburg et al. while Scherlag et al. showed that acute ischaemic injury to a His-Purkinje bundle in vitro induced conduction delays as well as a split in the His-Purkinje bundle potentials.18,21
Electrical Conduction in Purkinje Fibres
In normal human hearts, PF bundles provide the major route for rapid conduction at approximately 3–4 m/s of the impulse from the AV node tissues to the ventricular muscle. This is in contrast to much slower conduction in cardiac muscle (0.2–0.6 m/s).22 In 1972, Myerburg et al. reported on conduction of premature impulses in the ‘normal’ myocardium.23 They noted that the PF action potential duration (APD) increased progressively along the left bundle and reached a maximum value at an area they called ‘the gate’ situated at the intersection of the PF and papillary muscle.24 From this area onto the apex, the subendocardial PF APD shortened. In the mouse heart, conduction velocity (CV) in the midseptal region is reduced compared to proximal CV; the geometry of the bundle branches appears to be responsible for the reduced midseptal CV (Figure 1).25 This heterogeneity of APDs in the PF network prevents retrograde activation of PF from premature stimuli arising from the myocardium, serving as a functional ‘gate’.
When the described ‘normal’ PF APD heterogeneity is disturbed, the gate protection may be lost and reentry could occur. For example, cardiac ion channels in PCs are robustly remodelled during ischaemia and following MI, resulting in PF/PC APDs that are longer than their non-infarcted counterparts.26,27 In this remodelled substrate, premature impulses may conduct, but slowly and inhomogenously, blocking in several areas between the apex to the base to set up conditions for reentry. Similar findings would be expected to occur in the subendocardium of the left ventricle of failing hearts, where remodelling of the PC and associated prolongation of APDs are seen to be similar to that of post-infarcted PCs.27
Reentrant Arrhythmias in the His-Purkinje System
Reentrant arrhythmias are self-sustaining rhythm abnormalities that are distinct from disorders of impulse generation such as automaticity and triggered activity. Reentry occurs in the presence of anatomical or functional obstacles, allowing for the formation of circus movement that is similar to a closed loop circuit. First described by Mines in 1914, reentrant excitation requires three criteria:
- An area of unidirectional block of the propagating impulse in a potential pathway.
- Slowed conduction of the propagating wavefront allowing for sufficient time for substrate recovery and propagation of reentry (tissue size greater than arrhythmia wavelength, defined by action potential duration x conduction velocity).28
- Interruption of the reentrant circuit at any point along its path terminates the circus movement.
Thus, reentry is supported by the combination of reduced APD, reduced CV, or an increase in tissue size. In patients with structural heart disease (SHD), such as ischaemia, infarct and cardiomyopathy, the effects of electrical remodelling and scar formation leading to the conditions for reentry is intuitively easy to understand. This includes a wide spectrum of reentrant mechanisms, including BBRVT, interfascicular VT, and intrafascicular-mediated macro- and micro-reentrant VT.29–33 However, with an increasing recognition of the role the HPS plays in arrhythmogenesis, there is a growing body of literature showing similar reentrant mechanisms in structurally normal hearts.12,34–36
Bundle Branch Reentrant Ventricular Tachycardia
BBRVT is a macro-reentry circuit using the left and right bundle branches and myocardium connecting the two bundles. Typical BBRVT displays a left bundle branch block (LBBB) QRS morphology, resulting from anterograde conduction down the right bundle (RB), transseptal conduction across the interventricular septum and retrograde conduction up the left bundle (LB). Atypical BBRVT uses the same circuit in the reverse direction, exhibiting a right bundle branch block (RBBB) QRS pattern due to anterograde conduction down the LB and retrograde conduction up the RB.
BBRVT was initially described in patients with SHD and conduction system disease with prolonged HV interval, particularly in patients with dilated cardiomyopathy of both ischaemic and non-ischaemic cardiomyopathy patients.30,31 HPS disease (and the associated conduction delays along the fascicles and bundles) serves as the substrate for BBRVT, allowing sufficient time for recovery of refractoriness and sustained reentry. There has been increasing recognition of BBRVT occurring without SHD and in younger patients with normal biventricular size and function.37 In a series of six cases, all patients exhibited prolonged HV at baseline (with a mean 69.2 ms), with ECG evidence of conduction system disease apparent in four of the patients. Genetic testing identified mutations in the sodium voltage-gated channel alpha subunit protein coding gene (SCN5A) or lamin A/C protein coding gene (LMNA) in three of the six patients, resulting in isolated conduction system disease. Despite the normal biventricular structure and function, these patients presented with syncope and cardiac arrest, highlighting the malignant potential of BBRVT and the viable role in cases of unexplained sudden cardiac death.
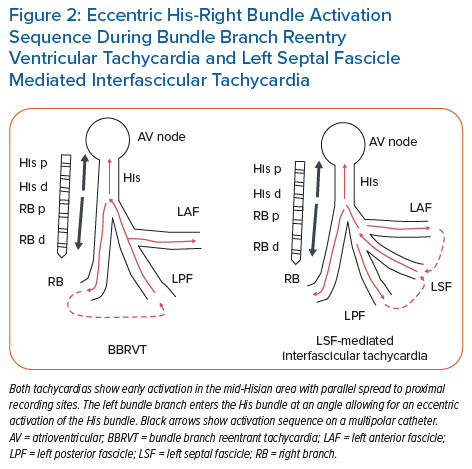
BBRVT should be considered in all cases of monomorphic VT, particularly if the surface ECG shows classic LBBB or RBBB QRS morphology during VT. At a minimum, His recordings should be obtained during sinus rhythm and tachycardia. Prolonged HV interval during sinus rhythm should alert the clinician to the possibility of BBRVT. During tachycardia, His activation is eccentric if both the His bundle and RB electrograms are encompassed by multiple electrode recordings (Figure 2), with HV intervals sometimes being the same but usually longer than intervals during sinus rhythm. His signals (H) preceding ventricular activation (V), with changes in H-H interval driving changes in V-V intervals strongly imply BBRVT, but do not exclude other fascicular mechanisms of VT, including interfascicular reentry.30,31 Confirmation of BBRVT must be made by verifying bundle-to-bundle reentry by:
- entrainment mapping of the RV apical region (in circuit) and RV base (out of circuit) affirming the RV apex as part of the macroreentrant circuit; or
- comprehensive recording of LB, RB and fascicular potentials, allowing identification of the retrograde (evident by retrograde conduction pattern and preceding His signals) and anterograde limbs exhibiting an anterograde conduction pattern and inscribed between His and V signals) limbs.
Ablation is a highly effective treatment for BBRVT and is the preferred approach for managing this disease.30,31,38,39 The RB is the ablation target in both typical and atypical BBRVT. Care should be taken to avoid mechanical injury to the RB during mapping given the proximity to the endocardium and propensity for temporary conduction block from local endocardial pressure. Ablation should be performed along the proximal portion of the RB, ensuring there is an adequate distance from the His bundle/AV node to avoid complete heart block. Following ablation, a repeat electrophysiology study should be performed to confirm lack of inducibility, particularly with long-short extrastimuli. Although ablation results in curative therapy, due to the advanced conduction system disease and SHD in most of these patients, implantation of an ICD may be needed.40–42
Intrafascicular Reentrant Tachycardia
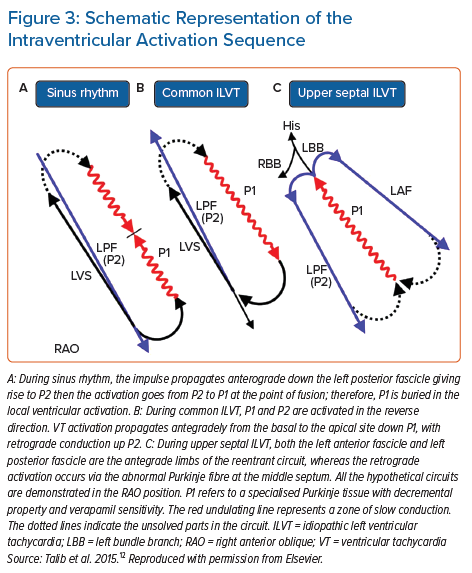
Intrafascicular tachycardia is synonymous with idiopathic left ventricular tachycardia (ILVT). Using the nomenclature from Nogami, the anterograde limb of tachycardia is termed P1 (which may be the septal fascicle), comprised of slowly conducting, abnormal PFs giving rise to diastolic potentials during tachycardia. The retrograde limb is termed P2, comprised of normal Purkinje/fascicular tissue giving rise to the presystolic Purkinje potentials (PP).35 Entrainment studies have confirmed reentry as the mechanism for ILVT.43
ILVT may be induced with both atrial and ventricular pacing.44,45 During sinus rhythm, anterograde conduction down P1 and P2 is present, but due to the slow conduction of P1, collision from the retrograde wavefront occurs from the distal connection of P1 with P2 (Figure 3). During ILVT reentry, anterograde conduction uses P1 with retrograde conduction over P2 (Figures 3 and 4). Previous studies have shown verapamil selectively affects P1 conduction without affecting P2.43 The typical VT morphology is RBBB with left axis deviation (LAD), due to involvement of the LPF and its associated myocardial exit.35 However, ILVT with RBBB and RAD QRS morphology may also be observed, resulting from tachycardia involving LAF and subsequent local myocardial exit.44
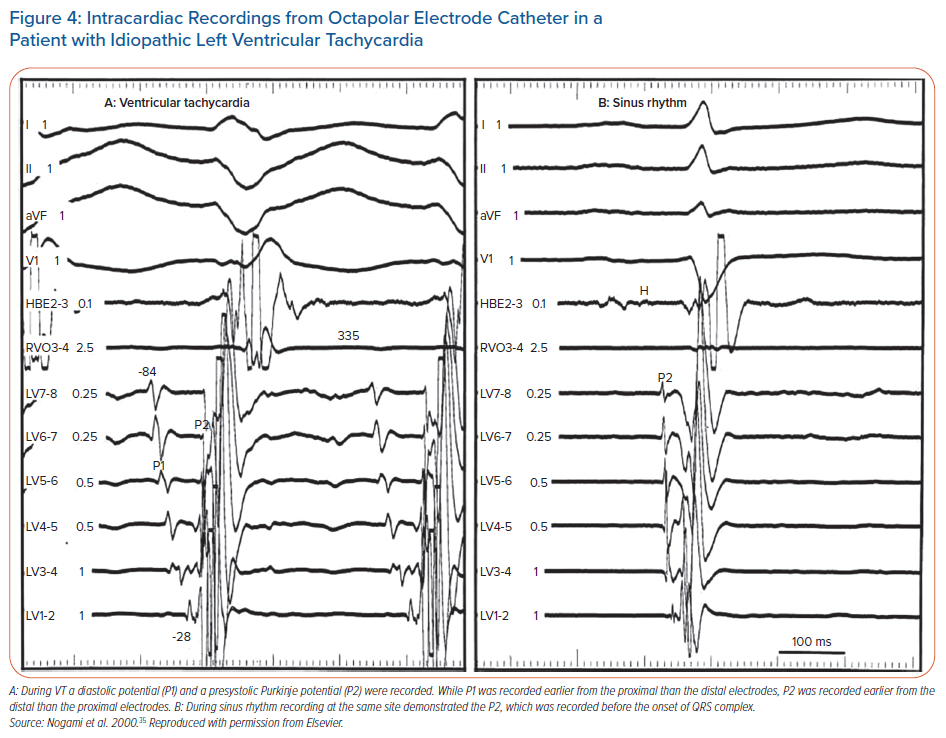
Analogous fascicular-mediated reentrant VT has been observed in a case series of four patients with either acute or remote MI with SHD, with successful ablation targeting diastolic Purkinje potentials (Purkinje-QRS interval of 58 ± 26 ms).46 It is unclear whether VT in these cases resulted from electrical remodelling due to ischaemia/infarct or whether it represents the same substrate as patients with ILVT coincidentally occurring in patients with acute/chronic infarct.
Originally, the reentrant circuit was thought to be contained within the Purkinje system, isolated from the surrounding myocardium.35 However, cases of verapamil-sensitive idiopathic LV tachycardia with RBBB superior axis VT have been reported, demonstrating participation of the LV septum in the circuit, sometimes without participation of the LPF in the tachycardia circuit.47,48 This shows that in some cases of ILVT, the LAF or LPF may act as a bystander, with myocardium serving as P2 or the retrograde limb of the reentrant circuit.
Recently, three cases of reverse-type left posterior fascicular VT (LPFVT) were described in a series of 242 patients with ILVT, with ECG characteristics of rSr’ morphology in V1, early precordial transition and inferior axis.49 Two of the three patients had concomitant common-type LPFVT. In all three cases, the electrophysiology study demonstrated the following:
- The left superior middle septum was the site of the earliest ventricular activation.
- Fragmented P1 signals were buried within the local ventricular electrogram.
- The P1 activation sequence demonstrated ‘retrograde’ conduction with apical to basal wavefront.
- P1 signals were linked to the subsequent LV septal signal.
Based on their findings, Phanthawimol et al. suggest P1 activates in a retrograde fashion opposite to anterograde conduction in common type LPFVT with myocardial exit in the intraseptum resulting in simultaneous exit in both RV and LV anterior septum that results in the rSr’ pattern in V1 (Supplementary Material Figure 1).
Oral verapamil may be effective in ILVT, particularly in patients with mild to moderate symptoms, but it is much less effective in patients with severe symptoms from VT.50 In a series of 37 patients, those with severe symptoms of ILVT ultimately required some form of non-pharmacological therapy, mostly with catheter ablation.50 Ablation is considered safe and appropriate, particularly in patients with severe symptoms or those who cannot tolerate anti-arrhythmic medications.
Several studies have shown high rates of acute ablation efficacy using various approaches, with long-term success rates varying from 70–90%.35,48,51–58 We previously detailed the published approaches for successful ablation of ILVT and refer the reader there for detailed discussion.59 Some key approaches for ILVT ablation include the following:
- Targeting the site of the earliest Purkinje potential during VT.
- Targeting the site of mid-diastolic Purkinje potential during VT.
- Ablation at or near VT exit sites or areas of earliest endocardial activation (especially in presence of PPs).
- Targeting sites of double potentials (presence of both P1 and P2) during VT.
- Empiric ablation perpendicular to the long axis of LV, particularly along sites of PP along the presumed, involved fascicle (when VT is not inducible).
- Targeting retrograde PPs observed during sinus rhythm (when VT is not inducible).35,48,51–56
Given the complexity and variation in HPS and Purkinje network, it is not surprising that there are likely variations in the anatomical structure and underlying VT circuit among various ILVT cases. In the studies mentioned above, each study required more than one technique to achieve successful ablation in a series of patients, highlighting the importance of understanding ILVT mechanism and having the flexibility to adjust the ablation strategy based on clinical findings rather than using a standard method.
In patients with inducible and sustained ILVT, we recommend careful mapping of both endocardial and Purkinje activation signals on two separate maps, paying close attention to the subtle timing differences in Purkinje signals. Care should be taken to avoid mechanical injury during mapping which may render VT non-inducible. In addition to activation mapping, entrainment mapping should be performed to confirm the location of circuit, with careful assessment of myocardial versus Purkinje pacing capture and signal return (recognising that some ILVT may involve myocardium in the tachycardia circuit).
In general, we recommend targeting P1 for ablation, highlighted by relatively slow, anterograde, mid-diastolic potentials. The junction of P1 and P2 may be targeted, but may require more extensive ablation, particularly when the LPF is involved. One should always be aware of the proximity to His and left bundles when ablating along the basal septum to avoid LBBB or complete heart block. In cases where VT is non-inducible, empiric ablation may be performed following previously described approaches.55,56 The anatomical approach consists of an ablation line perpendicular to the long axis of the LV at the midway point along the mid- to mid-inferior apical septum (targeting PPs).56 An alternative strategy includes mapping of the left-sided HPS and endocardial sinus breakout point, followed by a linear lesion perpendicular to the course of the LPF, 1 cm above the sinus breakout point. Although these studies evaluated patients with common ILVT involving the LPF (RBBB, LAD VT), one may consider an analogous approach in patients with ILVT involving the LAF who present with RBBB and RAD VT where VT cannot be induced.
Interfascicular Reentrant Ventricular Tachycardia
Interfascicular reentrant VT represents another form of macro-reentry using the HPS. Unlike BBRVT which uses both left and right bundles, interfascicular reentrant VT uses two of three left-sided fascicles and the intervening myocardium to create the reentrant circuit. This form of reentry using the fascicular system is most commonly observed in SHD, likely the result of electrical remodelling that results in slowed conduction necessary to sustain reentry.29,30,32,33,60 These cases usually involve the LAF and LPF acting as the anterograde or retrograde limbs of the VT circuit. Depending on the direction of the circuit, VT QRS morphology will show RBBB with LAD versus RAD, representing LPF versus LAF acting as the anterograde limb, respectively. VT morphology is the same as VT morphology seen in ILVT due to endocardial activation via fascicular exit.
Rare cases of interfascicular reentry have been observed in structurally normal hearts, exclusively using the septal fascicle as the retrograde limb, termed upper septal-dependent ILVT.12,36,61 These arrhythmias typically have narrow complex QRS morphology often with incomplete RBBB, which may often be mistaken for supraventricular tachycardia. During electrophysiology study, this form of ILVT may be distinguished from supraventricular tachycardia by a shorter HV interval compared to HV interval in sinus rhythm and eccentric His activation and AV dissociation. Upper septal ILVT may sometimes manifest following successful ablation of common ILVT with ablation at or near the LPF.36,62
The septal fascicle, located along the upper septum (between the LAF and LPF, often just distal to the left bundle), manifests P1 signals, but in retrograde activation during VT (Figure 3). This retrograde wavefront enters the main HPS, resulting in anterograde activation of the LPF and LAF, as well as eccentric activation of the His bundle (accounting for the shortened HV time compared to sinus rhythm; Figure 2) and anterograde conduction down the RB. The variability in QRS morphology is explained by the relative conduction times down the three bundles (RB, LPF, LAF). If myocardial exit is simultaneous along all three bundles, this would manifest with narrow QRS and normal axis. If there is an exit delay along the RB, LPF or LAF, the VT QRS would exhibit (incomplete) RBBB, LAD or RAD, respectively, or in various combinations of the above. Therefore, arrhythmias resulting from involvement of the septal fascicle are suspected when multiform fascicular tachycardia or alternate narrow and fascicular form are present at the same rates.36,63
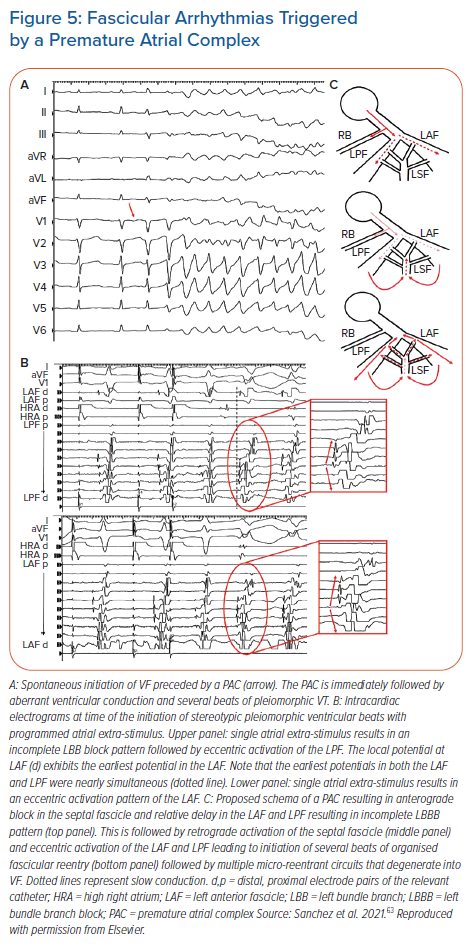
More recently, Sanchez et al. demonstrated a unique case of fascicular arrhythmias triggered by a premature atrial complex (PAC) and reproducibly degenerated into VF.63 All episodes were initiated by a PAC, which resulted in incomplete LBBB followed by multiform fascicular beats and then VF (Figure 5A). Following the PAC, there is LB delay with anterograde activation of the LAF and LPF, followed by eccentric activation of LAF and LPF preceding the fascicular beats (Figure 5B). These findings are best explained by PAC blocking anterogradely in the septal fascicle, with delayed conduction into the LAF and LPF producing LBB conduction delay (Figure 5C upper panel). This is followed by retrograde activation of the septal fascicle (Figure 5C middle panel), with eccentric and simultaneous activation of both the LAF and LPF (Figure 5C bottom panel) which results in several beats of fascicular reentry and VF. Tachycardia was eliminated with ablation of the septal fascicle.
These arrhythmias are sensitive to verapamil therapy, and catheter ablation is effective with low rates of major complications.36,62,64,65 Consider the following points when deciding on ablation:
- Search for retrogradely conducting P1 signals along the left upper to mid-ventricular septum;
- Activation mapping should be performed along the HPS in the diastolic period, tagging sites with PPs and identifying the earliest diastolic potential.
- Earliest endocardial activation is remote from the critical limb of tachycardia and ablation at these sites will not be successful. The same is true for ablation along any fascicles/bundles that are conducting signals anterogradely.
- Entrainment mapping may help identify a successful ablation site by identifying whether the P1 signal is part of the reentrant circuit. It is important to ensure capture of the P1 signal during attempted entrainment and measuring post-pacing interval to the Purkinje signal rather than myocardial signals. Concealed entrainment may be difficult unless pacing results in selective Purkinje capture only without local myocardial capture.
- Ideal sites of ablation including concealed entrainment with post-pacing interval equal to tachycardia cycle length, stimulus to QRS interval during entrainment equal to Purkinje to QRS interval (P-QRS) during tachycardia, and P-QRS of 50 ms or longer.
- Although upper septal ablation has been performed near the LB without proximal HPS injury, ablation should be performed more distally when possible to avoid injury to the His/left bundles.36,64,65
Purkinje–Myocardial Reentry
Bogun et al. showed that nine of 81 consecutive patients with previous MI and monomorphic VT demonstrated reentry using the Purkinje system, with all cases demonstrating QRS duration <145 ms.13 Although this macro-reentrant circuit mostly involved the myocardium looping around the area of MI scar, the Purkinje fibre was involved in the VT circuit and served as the target for successful ablation. Specifically, seven of the nine patients had successful ablation targeting the exit site from the Purkinje fibres following verification of PPs and concealed entrainment at that site. In two other cases, successful ablation was performed along the myocardium at the VT common pathway site. In these cases, electrical remodelling of the surviving PFs near regions of scarring resulted in enough conduction delay to allow reentry.
Clues for fascicular involvement in post-infarct VT include narrow QRS during VT and the presence of PPs preceding ventricular activation. Confirmation of fascicular involvement include changes in PP driving changes in VV during tachycardia cycle length changes and concealed entrainment from PP sites.
Conclusion
An increasing body of evidence has illustrated the various reentrant VT circuits involving the fascicular tissue in both structurally normal and abnormal heart, with overlap between the two. Despite the heterogeneity of location of the reentrant mechanism, the overall principles underlying each reentrant VT form are similar. A methodical approach to fascicular VT by incorporating our understanding of HPS anatomy and application of standard electrophysiological principles will help guide successful ablation.
Click here to view Supplementary Material.
Clinical Perspective
- The His-Purkinje system is a complex network of conduction fibres located throughout the ventricles, composed of specialised conduction cells called Purkinje cells.
- Reentry is one of several mechanisms that result in ventricular arrhythmias involving the His-Purkinje system. Enhanced automaticity and triggered activity are others.
- Reentrant arrhythmias of the His-Purkinje system consist of bundle-to-bundle reentry, interfascicular reentry, intrafascicular reentry and macro-reentry involving the myocardium. These reentrant arrhythmias may occur in both structurally normal and abnormal hearts.
- Catheter ablation is an effective tool in the treatment of reentrant arrhythmias in the His-Purkinje system.