Over the last few decades there has been increasing interest in local and global cardiac electro-mechanics. This is largely because of the recognition of the adverse effects of conventional right ventricular pacing (RVP) and beneficial effects of cardiac resynchronisation therapy (CRT) and novel pacing modalities, such as left bundle branch pacing (LBBP), His bundle pacing (HBP) and left ventricular septal (LVSP).1–5 While CRT is usually considered to consist of biventricular pacing (BiVP), it was already known in the 1970s that HBP could normalise the QRS complex of left bundle branch block (LBBB) patients.6 Such an effect can only be understood if the conduction block in LBBB is proximal, which was demonstrated elegantly by Upadhyay et al.7 More recently, LBBP was introduced as an easier way to capture the left bundle branch in LBBB patients,8 while LVSP (septal pacing without capturing the conduction system) also appeared to also provide excellent cardiac pump function.9,10
For proper understanding of the effect of ventricular dyssynchrony and its prevention or correction by a pacing therapy, insights into cardiac mechanics and energetics are imperative. After all, mechanical coordination between all muscle fibres determines total pump function as well as the amount of energy required for the contraction. Fortunately, information on cardiac mechanics is rapidly increasing because of the development of dedicated non-invasive imaging techniques for assessment of regional myocardial deformation (strain). Also, modern modalities like PET allow us to obtain information on regional myocardial metabolism. While in many studies the sole emphasis is on improving pump function, it may also be important to pay attention to the metabolic economy of the heart. After all, clinical studies have shown the potentially harmful effects of longer-lasting increases in myocardial energy expenditure through inotropic drug therapy.11
In this review we will address cardiac electro-mechanics and mechano-energetics during dyssynchronous and (re)synchronised activation. For this purpose, we review information from studies in animal experimental models and patients.
Electro-mechanical Coupling and Myocardial Efficiency
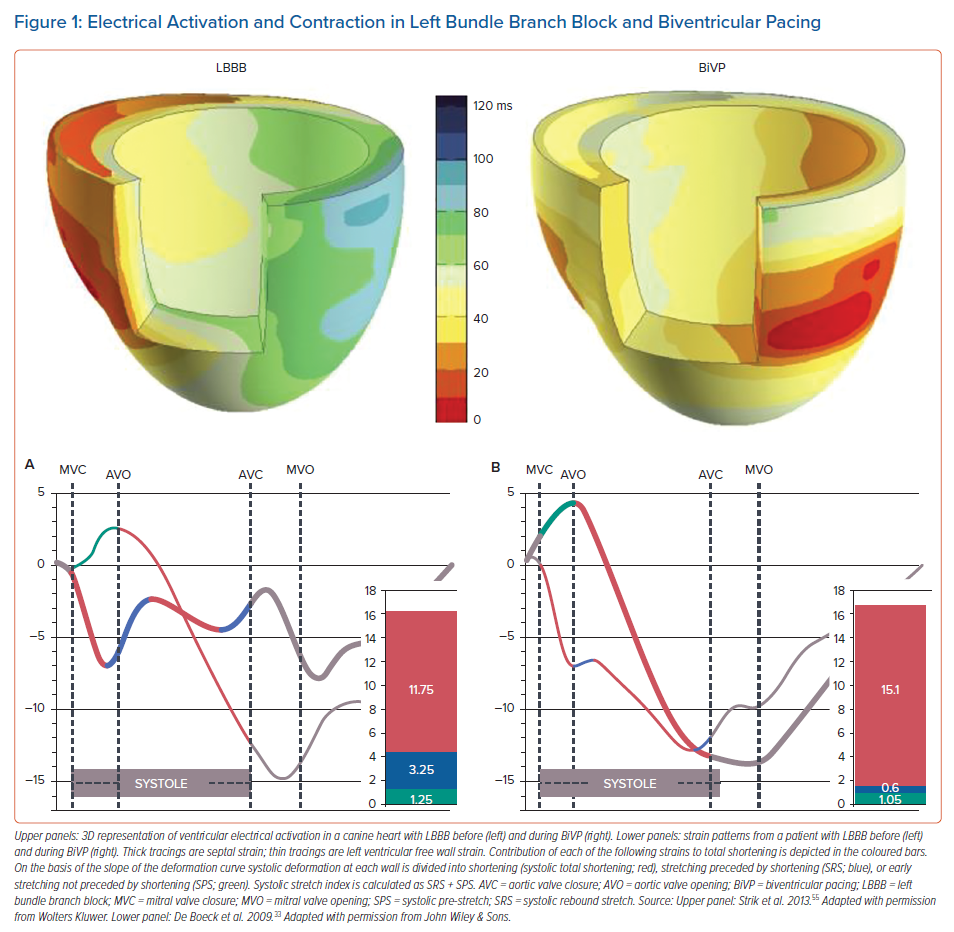
The sequence of electrical activation in the normal heart – as well as in the paced heart – is elaborated on in a related article in this volume.12 Like in any muscle, contraction of cardiac muscle cells is evoked by an action potential. The action potential triggers calcium influx that subsequently initiates calcium-induced calcium release. The time between the upslope of the action potential and binding of calcium to the myofibrils is approximately 30 ms. On a global basis this delay can be observed as the delay between the R wave of the ECG and the onset of left ventricular (LV) pressure rise. Because of this excitation–contraction coupling, it is not surprising that dyssynchronous electrical activation also leads to dyssynchronous contraction. In various studies in normal canine hearts, the onset of segment shortening proved to be closely related to the timing of electrical activation.13–15 Accordingly, regions with earliest electrical activation (red in left upper panel of Figure 1) also start to contract first (thick tracing in lower left panel of Figure 1).
However, this figure also depicts that shortening patterns are not just shifted in time, but also have a completely different morphology, indicating discoordination of contraction. These complicated regional differences in contraction pattern are most likely related to the local differences in myocardial fibre length during the early systolic phase, i.e. regional differences in effective preload. The discoordination is characterised by early systolic shortening in early-activated regions and coincident systolic pre-stretch (SPS; green in Figure 1) in late-activated regions. Later in systole, late-activated regions show pronounced shortening, while early-activated regions may be even stretched, referred to as systolic rebound stretch (SRS; blue in Figure 1).16–18 An index combining SRS and SPS is the systolic stretch index (SSI) where SSI = SRS + SPS).
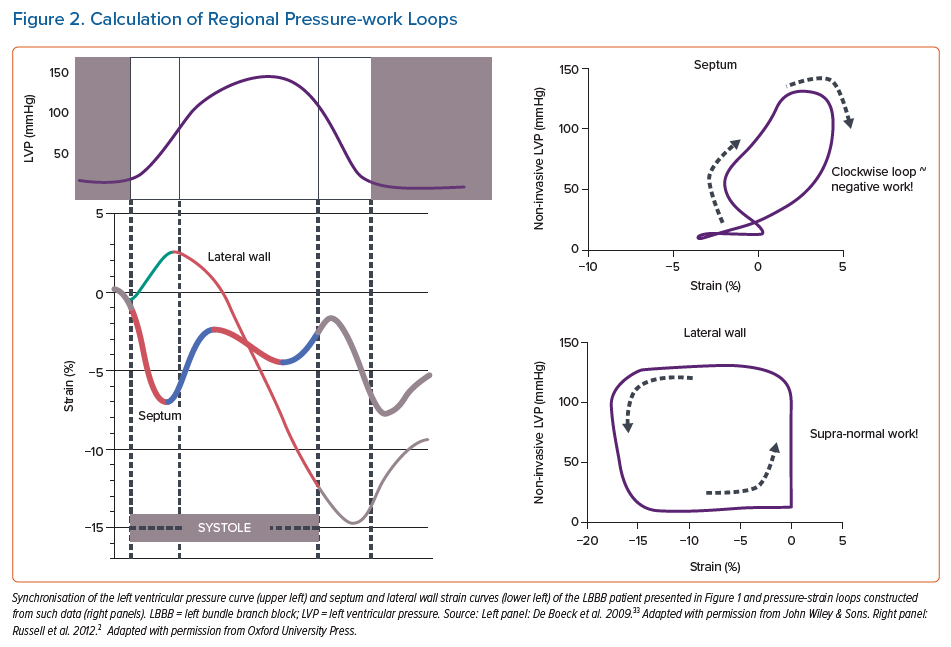
The functional meaning of these strain differences can be better appreciated when plotting strain as a function of pressure or stress. The area of the pressure-strain or stress-strain loops reflects external mechanical work of a particular segment. Figure 2 (right panel) shows that pressure-strain loops have a figure-of-eight shape in the early-activated septum of a LBBB heart and are enlarged in the late-activated lateral wall. In cases where significant septal rebound stretch is seen, loops in early-activated regions run in a clockwise direction, indicating that work is being performed on that segment rather than that it generates work. Russell et al. have called this behaviour ‘wasted work’.19 During considerable dyssynchrony, such as during RVP and LBBB, external work is close to zero, and often even negative in early activated regions and double the normal values in late activated regions.18,20
While originally myocardial pressure-strain loops required invasive LV pressure measurements, Russell et al. developed a non-invasive method to assess LV pressure.21 It was shown that peak systolic arterial pressure (measured using the arm-cuff method) in combination with timing of opening and closure of the cardiac valves (using echo-Doppler), provided a reliable systolic part of the LV pressure curve that can be used to plot strain against and thus non-invasively determine regional myocardial work.
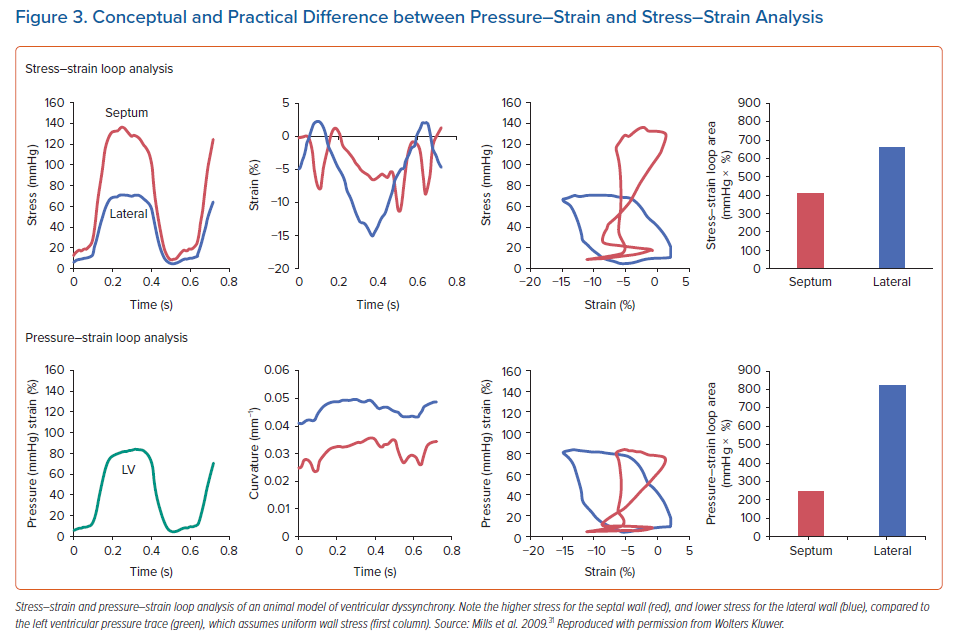
On the other hand, Duchenne et al. modified the invasive technique so that actual stress-strain loops could be determined. To this end, they used the dynamic curvature and thickness of the local wall segment and applied the LaPlace law.22 In later studies, the authors used non-invasive LV pressure data to construct stress-strain loops without the need for intra-arterial access.23 With this approach, stress-strain loops can also be safely determined in a clinical setting. An interesting observation by these authors is that by using the stress-strain approach they showed in an animal model of ventricular dyssynchrony that the distribution of local work became inhomogeneous acutely after inducing dyssynchrony, but almost normalised after 8 weeks of dyssynchrony. This relative normalisation of local mechanical work was mirrored by normalisation of the distribution of glucose uptake, measured using fluorodeoxyglucose-PET scan. In contrast, pressure-strain loops demonstrated less homogenisation in work, and a weaker correlation to glucose uptake.22 Because stress-strain loops correct LV pressure for the effect of the asymmetric remodelling and curvature induced by dyssynchrony – whereas pressure-strain loops do not – it is likely that they better represent the true work performed by the myocardium. Indeed, the septum of a remodelled, dyssynchronous LV is typically thin, and as a result, experiences relatively higher wall stress. In contrast, the typically thickened lateral wall thereby experiences relatively lower wall stress. The effect of the regional wall stress on the calculation of myocardial work is demonstrated in Figure 3.
Because local myocardial perfusion adapts itself to the oxygen demand (autoregulation), it is not surprising that during dyssynchronous activation myocardial blood flow, oxygen consumption and glucose uptake also differ between regions.16,17,24–28 Compared with sinus rhythm, myocardial blood flow and oxygen consumption are 30% lower in early-activated regions and 30% higher in late-activated regions.16,17,20,26 Furthermore, compared to a LV with normal conduction, dyssynchronous hearts show on average significantly higher glucose consumption for the same amount of performed regional myocardial work, supporting the notion that dyssynchrony leads to less efficient work.22
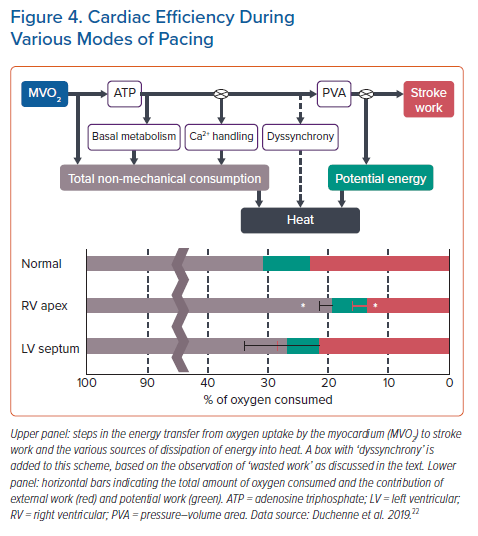
The discoordination in contraction also impacts overall myocardial efficiency, the amount of oxygen needed for a unit output of the cardiac pump. In anaesthetised open-chest and conscious dogs RV apex pacing decreased mechanical output, whereas myocardial oxygen consumption was unchanged or even increased compared to atrial pacing.29,30 Consequently, efficiency decreased by 20–30% in these studies.
A similar number has been reported by Mills et al., who determined mechanical energy using potential energy and stroke work from pressure volume relations and oxygen consumption by direct measurements.31 These investigators found that while in the normal heartbeat ~22% of all oxygen consumed is used for pump action, during RVP this amount drops to ~14%, so a ~30% reduction in efficiency (Figure 3). The figure also depicts that this reduction in efficiency by RV apex pacing is almost completely abrogated when applying LVSP (see below).31
Electro-mechanics During Biventricular Pacing
With the main messages of the previous paragraph in mind we now discuss to what extent BiVP, LVSP and conduction system pacing prevent or reverse dyssynchronous activation, depending on whether pacing is performed in a heart with narrow or wide QRS complex, respectively. Because BiVP has been performed for more than two decades, it is not surprising that considerably more information is available on this pacing mode than on LVSP, HBP or LBBP.
The primary application of BiVP is CRT and there is extensive literature on the change in strain patterns in hearts after starting biventricular pacing. When looking at gross level (difference between septum and LV lateral wall), BiVP clearly creates a more uniform strain pattern compared to RVP or LBBB (Figure 1, right panels). This has been demonstrated in animals (Duchenne using speckle tracking and Vernooy using MRI tagging) and in humans.20,22,32 DeBoeck et al. demonstrated that BiVP does not change the total amount of systolic deformation, but that it redistributes shortening from the LV lateral wall to the septum and decreases systolic stretch (mainly SRS in the septum, right panel Figure 1).33 These investigators also demonstrated that SRS was a more sensitive marker of the mechanical substrate for CRT response than the time interval between peak shortening in the various segments.33,34 More recent studies corroborated these findings by showing that the sum of postero-lateral systolic pre-stretch and septal rebound stretch, called SSI, is strongly associated with clinical outcome after CRT.35,36
Because SRS is tightly related to negative or very low external work, it is not surprising that also the normalisation of myocardial work distribution after CRT is related to the benefit of CRT.23 A prospective randomised trial was performed in 200 patients to evaluate the clinical usefulness of myocardial work to select patients who benefit from CRT.37 The investigators showed that the difference between myocardial work performed by septum and LV lateral wall at baseline predicted CRT response (defined as LV end-systolic volume reduction >15% after 6 months) with an area under the curve (AUC) of 0.77 (95% CI [0.70–0.84]). The predictive value was further increased by adding information on septal scar as obtained by magnetic resonance late enhancement in a subpopulation of patients (AUC 0.88; 95% CI [0.81–0.95]). Importantly, predictive value was as good in patients with QRS duration 120–150 as in the entire cohort. Equally important was the observation that work difference alone and combined with septal viability predicted long-term survival without heart transplantation with HR of 0.36 (95% CI [0.18–0.74]) and 0.21 (95% CI [0.072–0.61]), respectively.
What may make SRS, SSI and myocardial work good predictive measures is that they all are dependent on the contralateral septum-lateral wall interaction. Importantly, this mechanical behaviour of contralateral stretching and shortening is not only indicative for an electrical activation delay, it also strongly indicates good contractile function of the myocardium. Therefore, large SRS, SSI and septum-lateral wall work difference are all indicative of the combination of gross electrical septum-lateral wall dyssynchrony and good overall myocardial viability and contractile function.
Because abnormal systolic strains in dyssynchronous hearts are closely related to local energetics (see above), it is reconfirming that upon resynchronisation the distribution of blood flow, oxygen consumption and glucose uptake, as studied by PET, becomes more uniform.24,27,38,39 The finding that this recovery of blood flow occurs within 2 weeks after onset of CRT and disappears within 15 min after stopping CRT, indicates that the perfusion inhomogeneities in the LBBB hearts importantly relate to the abnormal distribution of workload (for example evidenced by the inhomogeneities in strain between septum and later wall).24,39 In addition, Knaapen et al. demonstrated that CRT also increases septal blood flow during adenosine infusion.39 This increase in septal flow was immediately abrogated upon stopping CRT, suggesting that CRT alleviates impediment of perfusion by the abnormal contraction pattern.39 Furthermore, an increase in hyperaemic flow was found to be associated with a decrease in eccentricity of LV hypertrophy, an index of wall stress.
The more uniform distribution of cardiac contraction during CRT also leads to a higher efficiency of the entire LV chamber. Nelson et al. showed that resynchronisation increases the maximum rate of rise of LV pressure while myocardial oxygen consumption even slightly decreased. In these patients a similar increase in the rate of rise of LV pressure, evoked by dobutamine infusion, significantly increased myocardial oxygen consumption.40 Kyriacou et al. showed that BiVP at optimal atrioventricular-delay increases both LV external work and oxygen consumption, but the increase in cardiac work was ~80% greater than the increase in oxygen consumption, signifying an improvement in cardiac mechano-energetics.41
Therefore, restoration of coordination of contraction by CRT improves cardiac function through a mechanism different from that by inotropic stimulation. The observation that CRT improves ventricular efficiency is important for two reasons. First of all, there is evidence that, in general, failing hearts have a reduced ratio between work performed and oxygen consumed (mechanical efficiency).42 Also, these hearts often have compromised energy metabolism, as evidenced by a reduced ratio of phosphocreatine to total adenosine triphosphate.43 So, any relief of the compromised energy metabolism can be expected to have a relevant benefit.
Very few therapies in heart failure improve cardiac efficiency. While inotropic drugs increase myocardial oxygen consumption in parallel with contractility, vasodilators decrease both parameters. The effect of phosphodiesterase III inhibitors depends on the vasodilative and inotropic effects as well.44
What is Known About Left Ventricular Septal, His Bundle and Left Bundle Branch Pacing ?
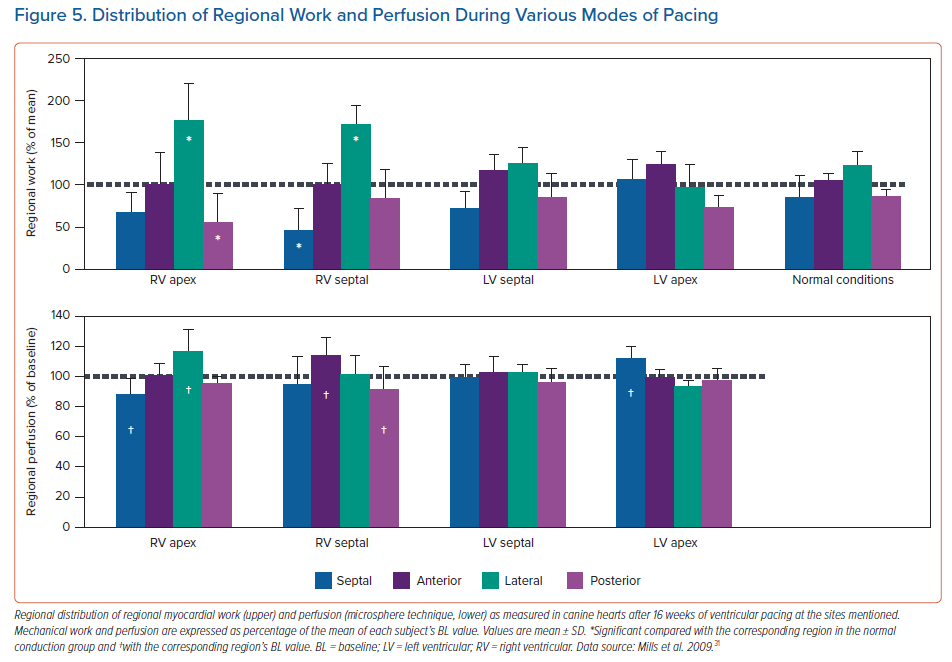
Until now there are no patient studies comparing the strain patterns or electro-mechanics during BiVP, LVSP, HBP and LBBP. The most detailed information on this topic has been obtained in animal studies on LVSP in comparison with normal conduction and RVP.31 In this study, MRI tagging was used to determine strain patterns. While during RVP circumferential strain, external work and blood flow were significantly lower in the septum and elevated in the LV free wall, such differences were not observed during LVSP and during normal conduction (Figure 4). These differences coincided with lower values of internal stretch fraction (a measure of mechanical discoordination) and peak shortening delay. Both at 1 hour and 16 weeks of pacing, myocardial efficiency (ratio of external work and oxygen consumption) was 30–40% decreased during RVP compared to normal conduction, whereas there was no decrease in efficiency during LVSP (Figure 5). Moreover, at the end of the 16-week experimental protocol, myocardial efficiency was compared between RVP, BiVP LVSP in the acute setting. Here efficiency was significantly higher during LVSP than during RVP, whereas there was no significant difference between LVSP and BiVP.
Zanon et al. investigated the distribution of myocardial blood flow during RVP and HBP in patients with standard pacemaker indication. They observed a significantly better perfusion score during HBP than during RVP, which was also shown by a more uniform distribution of blood flow.45 As myocardial blood flow and work are closely related, as discussed above, these data seem to corroborate the idea that HBP maintains a more uniform distribution of myocardial work. In a similar patient cohort
Tang et al. investigated regional LV function during selective HBP and RVP.46 These investigators found that during RVP the maximal time difference in peak strain between all segments increased from ~100 to ~180 ms, whereas during selective HBP this increase was less pronounced (~135 ms). While the latter data show that selective HBP did lead to some degree of desynchronisation, this is significantly less than during RVP. In the CRT application HBP has shown to provide a haemodynamic improvement that is at least as large as that of BiVP.10,47
In HBP (and also LBBP) the exact position of the pacing lead is important. When the lead is positioned exactly in the conduction system (‘selective’ HBP) low-voltage stimulation suffices to achieve a narrow QRS complex. However, higher voltages are needed in less optimal positions (non-selective HBP).48 Bednarek et al. investigated mechanical dyssynchrony using speckle tracking echocardiography in patients during selective and non-selective HBP.49 Speckle tracking images showed premature contraction in the basal septal segment only during non-selective HBP, but this was confined to the basal septum and basal inferior segment. Moreover, this delay was only ~50 ms, as compared ~30 ms during selective HBP, but much smaller than values over 100 ms, known to occur during RVP. This small degree of dyssynchrony also explains the lack of significant differences in LV global longitudinal strain, as measure of global LV function.
The only article so far to report speckle tracking measurements during LBBP compared LBBP to RVP and found shorter maximal time differences in peak strain during LBBP (66 ms) than during RVP (149 ms).50
Together these studies indicate that the degree of dyssynchrony (as assessed by time-to-peak shortening, strain patterns or internal stretch fraction) during conduction system pacing is moderate and close to physiological while significantly smaller than during RVP. These results also make a uniform distribution of myocardial work likely, but this needs to be confirmed by future studies, involving measurement of regional work, as described above.
Furthermore, while the above-mentioned publications relate to the use of LVSP and conduction system pacing in patients with bradycardia indication, normal LV ejection fraction and a narrow QRS complex, there is no data on the effect of these pacing modes on myocardial strains and work in patients with CRT indication. Theoretically the effect of conduction system pacing would depend on the function of the Purkinje system, especially in case of LBBP. Upadhyay et al. recently showed convincingly that in patients with LBBB who were referred for device implantation or substrate mapping the site of complete conduction block was in the His bundle in 72% and in the proximal bundle in 28%. HBP corrected wide QRS in 54% of all patients with LBBB pattern and 85% of those with complete conduction block.7
Moreover, LVSP and LBBP may at least partly take advantage of the rapid conducting endocardial muscle fibres. Indeed, several studies showed the benefit of HBP, LBBP and LVSP as alternative to CRT, as evidenced by reduction in QRS width, acute haemodynamic effect and long-term outcome.10,51,52 Therefore, more studies on the electro-mechanical and mechano-energetic consequences of LVSP, LBBP and HBP are needed to fully understand the benefits of these pacing strategies when applied with the purpose of resynchronisation.
Atrioventricular Delay in Resynchronisation
Beside resynchronising ventricular activation, any kind of CRT can also improve atrioventricular coupling. In many LBBB patients not only is intraventricular conduction delayed, but also atrioventricular conduction, as shown by a prolonged PR-delay. A combined clinical-computational modelling study indicated that up to two-thirds of the haemodynamic benefit of CRT may be caused by improving atrioventricular coupling and thereby preload.53 A patient study showed that CRT increased cardiac pump function more than proportionally compared to myocardial oxygen consumption, indicating that the combination of ventricular and atrioventricular resynchronisation can make the heart more efficient and yet increase pump function.41 While this study was performed using conventional BiV pacing, it is likely that a similar effect occurs during the more novel resynchronisation modes. Moreover, a recent study showed that BiV pacing in patients who are not CRT candidates but have a prolonged PR-interval improves ventricular stroke work, because of improved filling, further underlining the considerable effect of filling on cardiac pump function.54 In that study it was also suggested that HBP, LBBP and LVSP could be used for this purpose.
Conclusion
CRT has several beneficial effects on mechano-energetics of the heart: it alleviates impediment of the abnormal contraction on blood flow and increases myocardial efficiency. These two factors act together to increase the range of cardiac work that can be delivered by the patients’ heart, an effect that can explain the increased exercise tolerance and quality of life reported in several CRT trials. The positive effects of BiVP on mechano-energetics are established, but further confirmation is needed for LVSP, LBBP and HBP.
Clinical Perspective
- The efficiency of cardiac pump function (the amount of stroke work generated by a unit of oxygen consumed), appears to be an important determinant of long-term outcome.
- Cardiac efficiency is approximately 30% lower in dyssynchronous than in synchronous hearts.
- The novel left ventricular septal, His bundle and left bundle branch pacing minimise the amount of pacing-induced dyssynchrony at least as well as biventricular pacing.
- Biventricular pacing has been shown to increase cardiac efficiency when compared to conventional right ventricular pacing.
- Information on cardiac efficiency regarding the other pacing modes is limited to a large animal study showing the value of left ventricular septal pacing in maintaining efficiency.