With an ageing population and widespread use of implantable cardioverter-defibrillators, physicians are confronted with an increasing number of patients with symptomatic, drug-refractory ventricular tachycardia (VT). Catheter ablation is an important treatment option in the management of patients with structural heart disease and VT.1,2 In many patients, VT can be successfully ablated from the endocardium of the left or right ventricle via a trans-venous, trans-septal or retrograde trans-arterial access. However, even in high-volume centres, VT ablation from the endocardium fails in over one third of patients.3–5 One important reason for this high failure rate is that VT origin may lie in the epicardium which is not affected by endocardial ablation with current techniques.1
In 1996, Sosa first described a technique to obtain percutaneous access to the pericardial space in three patients with chagasic cardiomyopathy.6 Epicardial ablation has since been incorporated into VT ablation strategies by many centres7–10 with its use increasing in recent years.4 The addition of epicardial mapping and ablation to the VT ablation strategy in patients with failed, previous endocardial ablation, increases success rate.7–10 Epicardial access can be obtained in most patients by experienced operators. Nevertheless, epicardial mapping and ablation has its risks and the complication rate is higher than an endocardial approach.11
An important question regarding epicardial VT ablation, and the topic of this review is ‘in which patient should epicardial access be considered?’ Timing is important as well – most operators prefer to gain epicardial access before full anticoagulation is administered. Therefore, the decision on epicardial access generally has to be made before left ventricular endocardial ablation.
Several clues can suggest whether important epicardial substrate will be present in a given patient, and these will be discussed in the following sections. Some of these clues, like the type of cardiomyopathy and 12-lead electrocardiogram (ECG) of VT, are available before a first procedure; while intra-procedural findings, like electrogram characteristics and results of endocardial mapping, may help to define the strategy of future procedures in case of failure. In some instances, epicardial access is contra-indicated or, conversely, the only available option to ablate VT; this will be discussed in the last section.
Electrocardiogram and Electrogram Characteristics Predicting Epicardial Origin of Ventricular Tachycardia
Several groups have investigated the value of the 12-lead ECG of VT or electrogram characteristics for prediction of epicardial VT origin (see Tables 1 and 2).
Electrocardiogram Characteristics of Ventricular Tachycardia
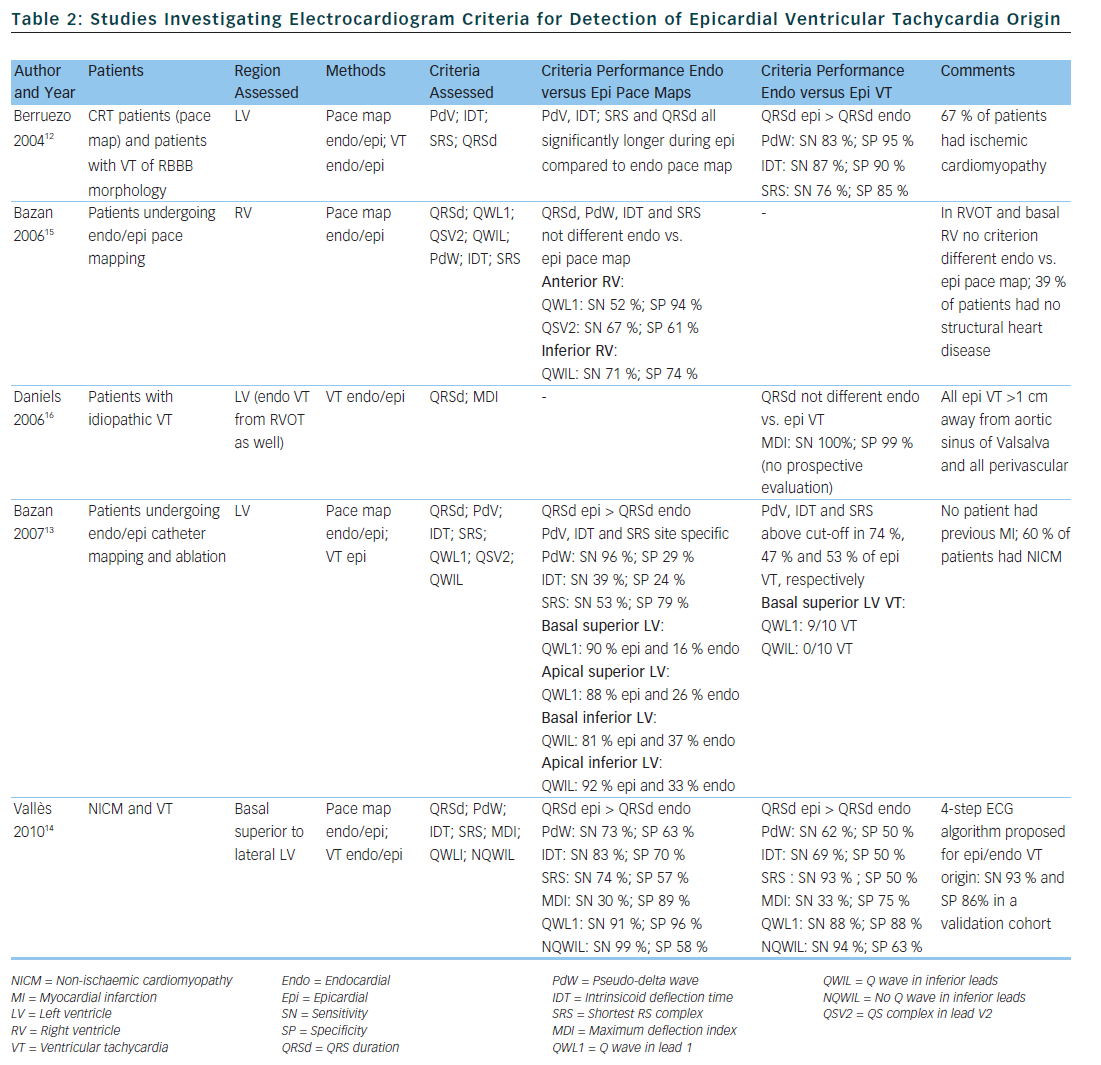
Berruezo et al. included patients with heart disease and VT with right bundle branch block morphology, and compared VT successfully ablated from the endocardium, with VT either successfully ablated from the epicardium or not successfully ablated from the endocardium.12 A pseudo-delta wave ≥34 ms, an intrinsicoid deflection time in V2 ≥85 ms and a shortest precordial RS complex duration ≥121 ms all suggested an epicardial origin of VT (see Tables 1 and 2). Later studies confirmed that these criteria are useful for distinguishing an epicardial origin for left ventricular VT, but sensitivity and specificity of the suggested cut off values were lower when applied to select areas of the left ventricle.13,14 Another study compared epicardial with endocardial paced beats and showed that these criteria did not apply to the right ventricle.15
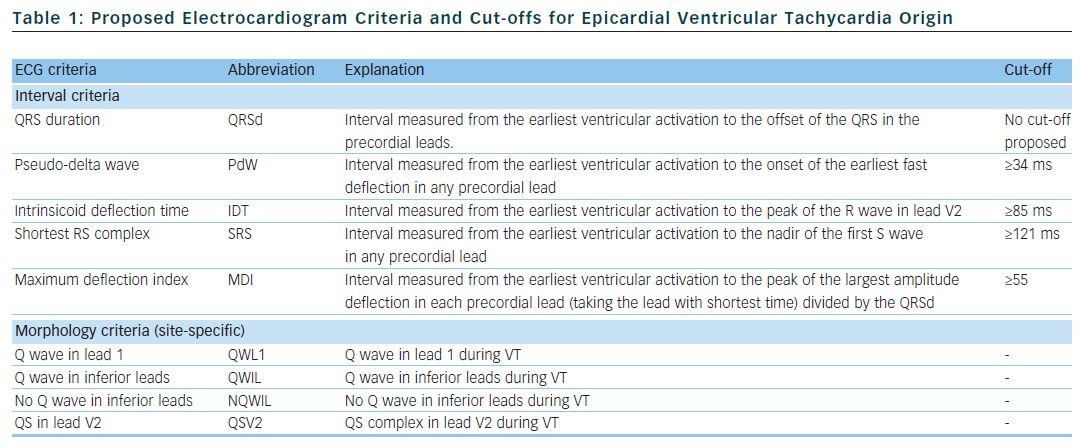
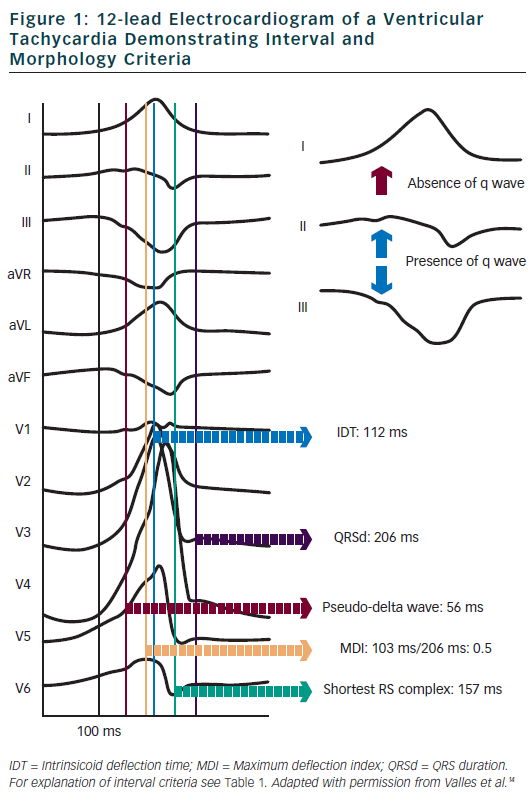
Daniels et al. reported on patients with idiopathic VT arising from the left epicardium, remote from the aortic sinus of Valsalva.6 Compared to the remaining patients with idiopathic VT, a maximum deflection index ≥0.55 identified patients with VT of epicardial origin (see Tables 1 and 2). Bazan et al. compared ECG features of paced beats at endocardial and epicardial right ventricular sites in a mixed population with and without structural heart disease.15 They found that a stimulus arising from the anterior right ventricular epicardium presents with a Q wave or QS complex in lead I and V2, respectively, and that a stimulus arising from the inferior right ventricular epicardium shows an initial Q wave in the inferior leads (see Table 2). In another study on 15 patients without prior myocardial infarction, the same group investigated QRS morphology during endocardial and epicardial left ventricular pace mapping and looked at QRS morphology of 19 epicardial VT.13 Site-specific morphology criteria that identified an epicardial origin were a Q wave in lead I for basal superior and apical superior sites, the absence of a Q wave in any of the inferior leads for basal superior sites, and a Q wave in inferior leads for basal inferior and apical inferior sites. Their proposed morphology criteria correctly identified epicardial origin in 84 % of VT (see Table 2). Vallès et al. tested most of the above-mentioned criteria in 14 patients with non-ischaemic cardiomyopathy, comparing endocardial and epicardial pace maps at basal superior to lateral sites in the left ventricle.14 They applied these criteria to VT mapped from the same region. Of all published criteria, the morphology criteria (presence of a Q wave in lead I and absence of Q waves in the inferior leads) were the most specific for both epicardial pace mapping and epicardial VT origin. The presence of a Q wave in lead I was also a very sensitive criterion (see Table 2). All interval criteria had modest specificity. They proposed a 4-step algorithm, including two morphology criteria and two interval criteria, with modified cut-offs. This algorithm, when applied to a validation cohort, had high specificity and sensitivity in determining epicardial versus endocardial VT origin in patients with non-ischaemic cardiomyopathy and VT from basal superior to lateral origin.14
The value of QRS duration in differentiating endocardial from epicardial origin has also been investigated in these studies. It was reported to be significantly longer in left ventricular VT of epicardial compared to endocardial origin12,14 and in epicardial compared to endocardial pace mapping.13,14 However, because of considerable overlap between groups, QRS duration was not able to differentiate epicardial from endocardial VT origin. On the other hand, QRS duration was not different in epicardial compared to endocardial idiopathic VT arising remote from the aortic sinus of Valsalva16 and in epicardial versus endocardial right ventricular pace mapping.15 The latter may be explained by the thinner right ventricular myocardium and less extensive Purkinje network in the right ventricle.
In summary, although some ECG criteria suggest an epicardial VT origin, other factors significantly influence their validity; most importantly the type of cardiomyopathy and region of VT origin. These limitations have to be considered when applying the above-mentioned ECG criteria for assessment of VT origin.
Electrogram Characteristics
The first clue to an epicardial substrate is the absence of low-voltage areas or fractionated/abnormal electrograms during sinus rhythm mapping; i.e. no diseased endocardial tissue. A VT activation map that shows a pseudo-focal activation pattern with the earliest endocardial signals barely preceding QRS onset also suggests a deep sub-endocardial or epicardial substrate. Other important tools that can help determine the presence of an epicardial substrate are endocardial unipolar voltage mapping and epicardial mapping through the coronary venous system.
Two studies by the same group tested the hypothesis that endocardial unipolar voltage mapping in patients with VT might identify the extent of epicardial bipolar voltage abnormalities.17,18 In both studies, the group first defined normal endocardial unipolar electrogram amplitudes in patients without structural heart disease. For the right ventricle, 95 % of endocardial unipolar signals had an amplitude >5.5 mV and defined a normal unipolar electrogram amplitude.18 For the left ventricle, the corresponding amplitude was >8.27 mV.17 Next, endocardial and epicardial bipolar voltage maps and endocardial unipolar voltage maps were generated in patients with mainly arrhythmogenic right ventricular cardiomyopathy/dysplasia (ARVC/D)18 and non-ischaemic cardiomyopathy.17
The ARVC/D patients had limited endocardial bipolar voltage abnormality, however, the area of unipolar voltage abnormality (cut-off <5.5 mV) was more extensive and correlated well with the larger area of epicardial bipolar voltage abnormality with respect to size and location.18 Importantly, in control patients with normal right ventricular endocardial unipolar voltage maps, epicardial bipolar voltage maps showed no abnormality.18
In patients with non-ischaemic cardiomyopathy and normal endocardial bipolar voltage maps but epicardial bipolar voltage abnormality, confluent regions of endocardial unipolar low-voltage areas (cut-off <8.27 mV) were observed in 82 % of patients, directly corresponding with areas of epicardial bipolar low voltage (61 % overlap).17 Control patients with structurally normal hearts and no epicardial bipolar voltage abnormality had no endocardial unipolar low voltage areas. Therefore, unipolar endocardial voltage maps can provide valuable information about the presumed extent of epicardial substrate, but so far this has only been studied in patients with ARVC/D and non-ischaemic cardiomyopathy.
The value of epicardial mapping through the coronary venous system in patients with sustained ventricular tachycardia was first described in 1998.19 Reithmann et al. reported their experience with coronary venous mapping using a micro-electrode catheter in 33 consecutive patients with structural heart disease admitted for VT ablation.20 Coronary venous mapping revealed fractionated diastolic electrograms during VT in 24 % of patients. Endocardial ablation in these patients was successful in 13 % compared to 72 % of patients in whom no epicardial diastolic electrograms were mapped during VT. Epicardial ablation was successfully performed in four of seven patients with unsuccessful endocardial ablation and diastolic potentials mapped via the coronary venous system. In summary, the coronary venous system provides an easy access to the epicardium. With a micro-electrode catheter a sizeable portion of the epicardium can be mapped and provide insights into the presence of an epicardial substrate.
Epicardial Substrate According to the Type of Cardiomyopathy
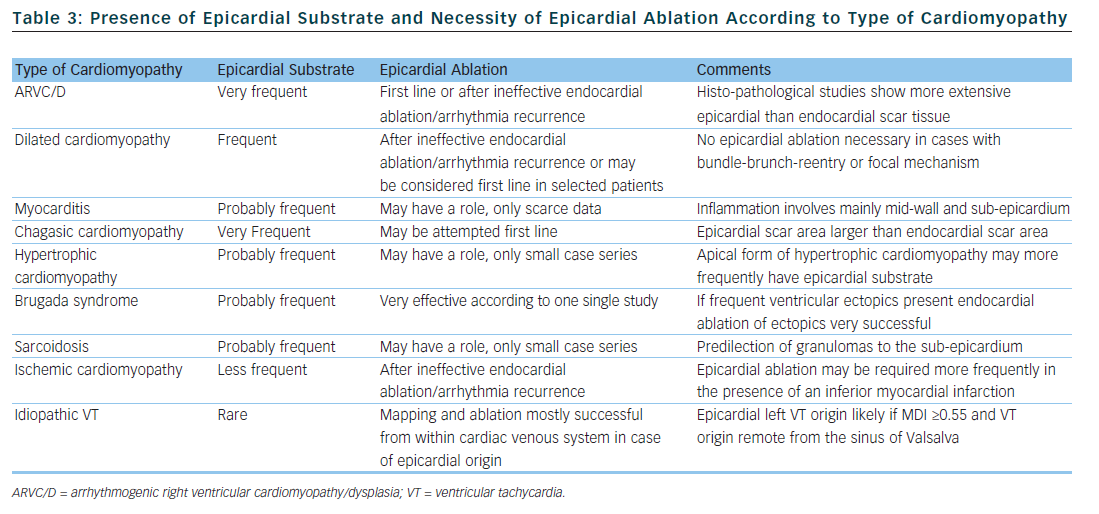
The substrate responsible for VT origin varies widely among different types of cardiomyopathy. Consequently, the rate by which successful VT ablation requires epicardial mapping and ablation is highly dependent on the type of cardiomyopathy. Of all the information available prior to a first VT ablation procedure, the type of cardiomyopathy is probably the best predictor for the necessity of an epicardial approach and we will discuss this in the next section. Table 3 summarises the available data with regard to epicardial substrate and type of cardiomyopathy.
Arrhythmogenic Right Ventricular Dysplasia
Attempts at endocardial VT ablation in patients fulfilling taskforce criteria for ARVC/D were disappointing, with high recurrence rates despite multiple procedures irrespective of acute procedural success.21 In a study by Verma et al., arrhythmia-free outcome was somewhat improved with a substrate-based endocardial ablation strategy, but after years half the patient experienced arrhythmia recurrence despite continuing their anti-arrhythmic drug treatment.22
Histo-pathological studies in the hearts of ARVC/D patients showed transmural myocardial atrophy with fatty or fibro-fatty infiltration that was much more extensive on the epicardial side.23 Accordingly, concomitant endo- and epicardial mapping in ARVC/D patients revealed a significantly larger epicardial area of electrogram abnormalities.24,25 Different groups have reported high rates of arrhythmia-free outcome with combined endo- and epicardial substrate ablation in ARVC/D patients.24–26 Two non-randomised, multicentre studies compared outcomes of ARVC/D patients treated with combined endo-epicardial ablation versus exclusive endocardial ablation; both found significantly higher long-term freedom from recurrent VT in the groups treated with combined ablation.27,28
Because of the unique pathology of ARVC/D, epicardial scar plays a major role in VT origin; important parts of re-entry circuits may lie epicardially in this patient population.23,24 Therefore, if VT ablation is contemplated in ARVC/D patients, an epicardial approach may be considered during the first procedure, following ineffective endocardial ablation, or following arrhythmia recurrence after an initially successful endocardial ablation. As discussed above, the extent of the epicardial substrate in ARVC/D patients may be identified by endocardial unipolar mapping.18
Dilated Cardiomyopathy
The main mechanism of VT in patients with dilated cardiomyopathy is myocardial reentry. Bundle branch re-entry, inter-fascicular re-entry and focal automaticity are rarer mechanisms.29,30 While endocardial ablation of myocardial reentry has a modest success rate, it is very successful for the remaining tachycardia mechanisms.29,30 Scar tissue is uniformly present in cases with myocardial re-entry and mostly located next to a valve annulus.30–33 The scar area may be larger in the epicardium than the endocardium or limited entirely to the epicardium in some patients. Contrast-enhanced cardiac magnetic resonance imaging of patients with dilated cardiomyopathy shows mid-wall fibrosis in up to one third of cases, different to the subendocardial/transmural pattern found in ischaemic cardiomyopathy.34,35 The presence of mid-myocardial fibrosis has been found to be predictive of an arrhythmic event.34 Intracardiac echocardiography can identify increased echogenicity in the lateral wall of the left ventricle (LV) of some patients with dilated cardiomyopathy.36 This increased echogenicity was found to correspond to abnormal epicardial substrate and therefore may guide an epicardial approach. As discussed above, the extent of the epicardial substrate in patients with dilated cardiomyopathy can also be estimated with endocardial unipolar voltage mapping.17
Combined endo- and epicardial ablation in patients with dilated cardiomyopathy and VT results in high rates of arrhythmia-free outcomes.30,31,33 However, the patient populations in these studies are highly selected as most had failed previous endocardial VT ablation, favouring the presence of an epicardial circuit. Results, therefore, cannot be generalised to all patients with dilated cardiomyopathy and VT, although epicardial ablation should be considered in patients with failed endocardial ablation.
Myocarditis
Except for chagasic myocarditis, there are only a few case reports and one study on VT ablation in patients with myocarditis. In that study, 20 patients with active, biopsy-proven myocarditis and VT underwent endocardial VT ablation.37 Epicardial ablation was performed during a second procedure in six patients because of procedural failure of endocardial ablation. Two patients (10 %) had arrhythmia recurrence during 28 months of follow-up. In patients with active myocarditis, contrast-enhanced cardiac magnetic resonance imaging showed a mid-wall and sub-epicardial distribution of inflammation, which suggests that the epicardium may be an important ablation target.38,39 However, VT will cease spontaneously during recovery in many patients with myocarditis, therefore the current data does not allow us to draw any conclusion about the role of epicardial ablation in this patient population. A total of five patients with chronic myocarditis referred at our institution for VT ablation required epicardial ablation. Scar area were confined to the subepicardium of the left lateral LV wall in all and inferior wall in two of them.
Chagasic Cardiomyopathy
The method of percutaneously accessing the pericardial space for epicardial VT ablation was first described in three patients with chagasic cardiomyopathy.6 Using combined endo- and epicardial access for VT ablation, the same group reported a high arrhythmia-free success rate.40 It was later demonstrated that patients with chagasic cardiomyopathy and VT have a two-fold greater epi- than endocardial scar area.41 Combined epi- and endocardial substrate ablation was very successful in reducing the number of recurrent arrhythmia in this study .
Hypertrophic Cardiomyopathy
Patients with hypertrophic cardiomyopathy may have monomorphic VT. According to published case series, epicardial substrate may be present in up to 80 % of patients, and epicardial ablation can terminate VT or prevent VT recurrence in a substantial number of patients.42–44 In patients with apical hypertrophic cardiomyopathy epicardial ablation may be especially useful.45
Brugada Syndrome
Triggers initiating polymorphic VT and ventricular fibrillation in Brugada syndrome patients can be ablated very successfully from the endocardium of the right ventricular outflow tract or the right ventricular Purkinje system.46 In nine Brugada syndrome patients with recurrent ventricular fibrillation but without frequent ventricular ectopics, mapping studies found pathological substrate located over the anterior right ventricular outflow tract epicardium.47 Extensive epicardial ablation of this substrate rendered most patients non-inducible with normalization of Brugada-type ECG patterns and very high long-term arrhythmia-free success rate.
Sarcoidosis
The first case series described results of ablation in eight patients with VT and sarcoidosis.48 In two patients, endocardial ablation failed to abolish VT; subsequent epicardial mapping showed an extensive epicardial substrate in one patient. Of note, four out of these eight patients ultimately required heart transplantation for recurrent VT. Another case series on nine patients with cardiac sarcoidosis reported one patient who underwent successful epicardial ablation after no endocardial substrate was found.49 Necropsy findings in patients with cardiac sarcoidosis show a predilection of granulomas to the sub-epicardium and interventricular septum.50
Coronary Heart Disease
Large, multicentre studies on the outcome of endocardial VT ablation in patients with predominantly coronary heart disease consistently report an arrhythmia-free success rate of approximately 50 % and a significant reduction of the number of VT episodes in the remaining patients.3,5,51–53
Due to the myocardial vascular supply, myocardial infarction causes sub-endocardial or transmural scars, a pattern that is also found on contrast-enhanced magnetic resonance imaging.35 High-density electro-anatomical mapping studies in patients with ischaemic cardiomyopathy report the endocardial scar area to be significantly larger than the epicardial scar area.33,54 However, early surgical studies already described that VT in ischaemic cardiomyopathy may involve the sub-endocardium and is amenable to epicardial interventions.55,56 Sosa et al. were the first to show the feasibility of epicardial ablation in post-infarct patients.57 Several studies have since reported good success rates of combined endo- and epicardial ablation in patients with ischaemic cardiomyopathy, mainly after failed endocardial VT ablation.7–9,33 Detailed endo- and epicardial mapping of stable VT circuits in post myocardial infarction patients revealed a putative epicardial isthmus in a minority of them.58 The prevalence of an epicardial circuit may be higher in patients with inferior or infero-lateral rather than anterior myocardial infarction.1,9,59 Therefore, epicardial VT ablation in patients with ischaemic cardiomyopathy should be considered after failed endocardial ablation. Because ischaemic cardiomyopathy is by far the most frequent cardiomyopathy present in patients with VT, this patient population will also be the largest in absolute numbers in whom an epicardial access is necessary for successful VT abolition.
Idiopathic Ventricular Tachycardia
Up to 14 % of outflow tract VT originate from the great cardiac vein and can be successfully ablated from within a cardiac vein or via a direct epicardial approach.60,61 Daniels et al. described ECG characteristics of idiopathic VT of epicardial origin, remote from the aortic sinus of Valsalva and compared them to idiopathic outflow tract VT.16 A myocardial deflection index ≥0.55 (MDI, see Table 1) was 100 %
sensitive and 98.7 % specific for an epicardial origin (see Table 2). Origin of VT was perivascular in all cases and ablation was successful in the majority, either through the coronary veins or via a direct epicardial approach. Another group described four patients with idiopathic epicardial VT originating from the crux of the heart.62 All patients had a MDI ≥0.55 and ablation was successful from the coronary veins in one and via a direct epicardial approach in two. According to another study, the MDI had limited value for discriminating endocardial from epicardial VT origin in sites adjacent to the left sinus of Valsalva like the distal great cardiac vein and anterior inter-ventricular cardiac vein.63 In patients with idiopathic VT a perivascular origin has to be considered and mapping and ablation from within the cardiac venous system is feasible and successful.
Other Considerations, Contraindications and Complications
In some instances, endocardial VT ablation is not possible because of left ventricular thrombus, mechanical valves in aortic and mitral positions, or severe peripheral artery disease that prohibits retrograde trans-arterial access. In such cases, epicardial ablation without simultaneous endocardial access is a possible alternative before considering surgical options.
Contraindications
In patients with prior pericarditis or cardiac surgery, pericardial adhesions that prohibit epicardial access or constrain catheter manoeuvrability can be anticipated and we prefer not to obtain percutaneous epicardial access in these patients.64,65 Similarly, pericardial adhesions may be present after prior epicardial ablation with subsequent pericarditis.64,66 Although other groups have reported that these pericardial adhesions can be dissected with the ablation catheter and a steerable sheath, we are very cautious about this as it may have devastating consequences.65,66 If epicardial access cannot be obtained or in case of adhesions, a direct surgical epicardial approach creating a sub-xiphoid pericardial window with surgical dissection of pericardial adhesions is our preferred alternative.67 Pericardial agenesis is a very rare disease precluding epicardial access. This diagnosis may be suspected from the ECG which shows right axis deviation and clockwise rotation of the QRS complexes in the precordial leads as well as from the chest x-ray which may demonstrate levo-position of the heart and elongation of the left heart border. Finally, the coagulation status and the presence of antithrombotic or anticoagulant drugs should be considered before attempting epicardial access. In the presence of systemic anticoagulation pericardial puncture should be postponed if possible.
Complications
Pericardial bleeding is the most frequent complication of pericardial puncture and can be present to some degree in up to 30 % of patients.1,11 Hepatic or intraabdominal bleeding may also occur after accidental puncture of the liver or a subdiaphragmatic vessel.68 Injury to the coronary arteries or the phrenic nerve by nearby ablation are other well recognised complications of epicardial ablation.11,68 It is important to remember that air in the pericardial space can increase the defibrillation threshold and should be evacuated before attempting defibrillation.69 Pericarditic symptoms have been reported in up to 29 % of patients after epicardial ablation and may manifest as major pericardial inflammatory reaction in some patients.11,68
Summary
Several clues may suggest the presence of epicardial substrate involved in a VT circuit and help with the decision to gain pericardial access. The most important factor is the type of cardiomyopathy. An epicardial substrate is frequently present in cases with ARVC/D or dilated cardiomyopathy. Electrocardiogram characteristics may have a role, but it is important to recognise their limitations. Both interval and morphology ECG criteria have been assessed for selected patient populations and morphology criteria also are site-specific. Electrogram characteristics and endocardial maps can provide additional evidence for the presence of epicardial substrate and should also be considered. Finally, pericardial adhesions may be present in patients with prior cardiac surgery or pericarditis and prohibit percutaneous pericardial access.








