There is growing evidence for the role of extrapulmonary vein (extra-PV) substrate in AF; that is, atrial fibrosis is a key pathophysiological player in the progress of AF. Prof Hans Kottkamp of Hirslanden Hospital in Zurich, Switzerland, highlighted the research he and his colleagues have been carrying out in this area. Atrial fibrillation does not beget atrial fibrosis, nor is atrial fibrosis related to increased age of the patient.16
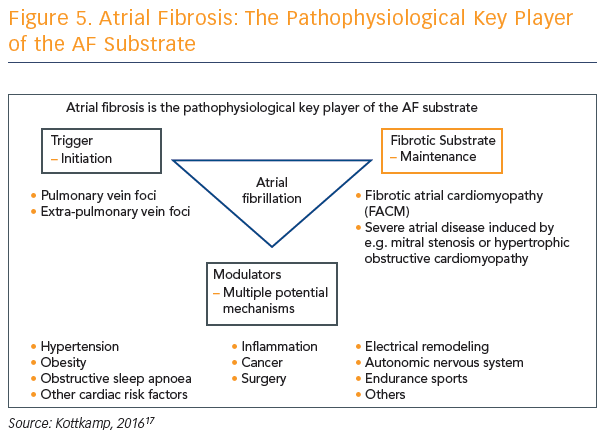
Atrial fibrosis is a complex process involving triggers, fibrotic substrate and modulators, and may be caused by multiple potential mechanisms. However, Prof Kottkamp says that it is becoming more and more clear that atrial fibrosis is the pathophysiological key player of the AF substrate, but we simply do not agree where it comes from (see Figure 5). Prof Kottkamp hypothesises that arrhythmia is a manifestation of fibrotic disease and not the other way around, but acknowledges this concept is controversial.
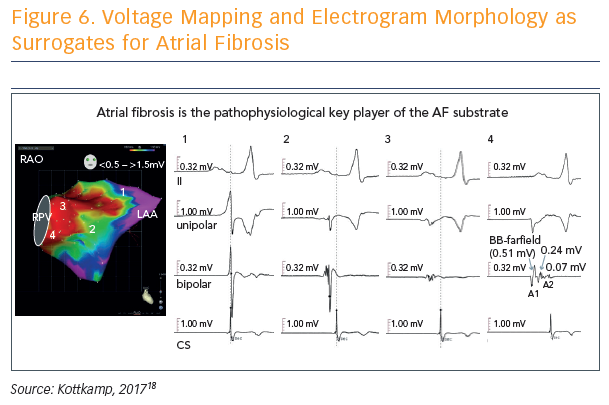
Voltage mapping and electrogram morphology can be used as surrogates for atrial fibrosis, and used to determine a fibrotic atrial cardiomyopathy (FACM) score: FACM 0 = purple (voltage >1.5 mV), FACM 4 = almost all parts red (voltage <0.5 mV) (see Figure 6).18 The fourth score, dubbed the strawberry, is the most massively and diffusely affected left atrium. “When this is affected, whatever you do, you will not get the patient back to sinus rhythm,” said Prof Kottkamp.
What, then, is to be done with respect to extra-PV substrate in AF? Prof Kottkamp discussed approaches depending on the presentation of the patient. For example, in a patient with persistent AF who has FACM 0, only a PVI is performed. In contrast, for FACM 1, 2 and 3 patients, box isolation of fibrotic areas performed in the first ablation procedure has led to good results.18
Prof Kottkamp has also been working with a new ‘globe’ ablation technology that may help combine stable, multi-electrode contact mapping with ablation in a single tool, and he feels it shows promise with respect to treating atrial fibrosis in the context of AF.
How Can We Explain Recent Trials Indicating No Benefit of Extra-PV Ablation?
Prof Gerhard Hindricks from University of Leipzig began his presentation by describing the different treatment strategies for extra-PV ablation. Durable PVI is the cornerstone of treatment strategies for the clear majority of patients with the various forms and presentations of AF. Add-on strategies that have been evaluated include deployment of linear lesions, complex fractionated atrial electrograms, AF termination, ablation of focal targets and left atrial appendage isolation, rotor detection and ablation, ablation of cardiac ganglia, and ablation of renal nerves. The latter three strategies were not discussed in detail, in favour of the classic moulds of extra-PV ablation.
In reviewing the treatment results, Prof Hindricks focused on several outcome measures, with the primary one being freedom from AF recurrence after ablation. Secondary outcomes parameters include complications, procedural data and resource utilisation. For all treatment strategies, positive effects on the main outcome measure (i.e. reduced AF recurrence rate attributed to the use of the extra-PV ablation strategy) can be found in the literature. However, whenever the treatment strategies had been evaluated in the setting of larger, multicentre studies, structured meta-analyses, or in the few randomised clinical trials available, most add-on strategies have failed to give solid and significant benefits.
After 20 years of AF ablation, no treatment strategy for extra-PV ablation received a recommendation in recent ESC guidelines for catheter ablation of AF, said Prof Hindricks. There is no strong evidence to advocate anything beyond PVI for any subclass of AF population, aside from solid PVI.
When interpreting the current clinical results, it is important to understand some key points and questions:
- Therapy allocation was mainly based on ECG phenotyping.
- There was no personalised approach in most studies.
- Most studies have been too small and underpowered.
- Most studies were carried out without clearly defined endpoints.
- Is the effect of additional ablation the same for all patient entities with AF?
- Did we induce both benefit and harm with a net-to-neutral effect?
- Did we apply acceptable standardisation of treatment strategies and follow-up?
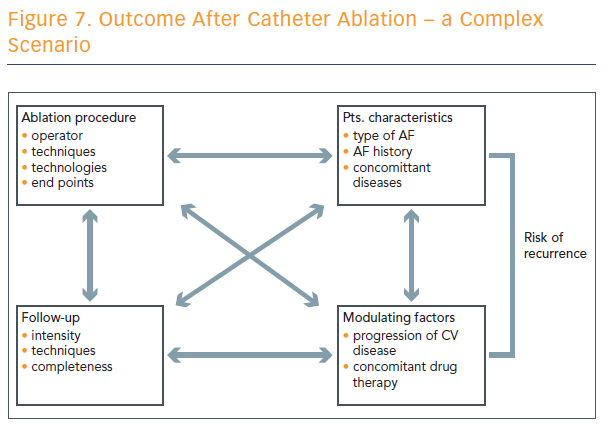
Outcome after catheter ablation is a complex scenario, and numerous factors play into the risk of recurrence (see Figure 7).
“We have to realise that the technique applied is an important part of the whole set-up that defines the recurrence rate, but it’s just one part among various factors that define the risk of recurrence after catheter ablation of atrial fibrillation,” concluded Prof Hindricks.
Is There an Optimal Extra-PV Substrate Ablation Strategy?
Procedural success for AF is affected by a range of factors – the type of AF, the substrate of the AF, if there is scar tissue, triggers, gender, the presence of valvular disease, left atrial size, and the technique. Dr Amin Al-Ahmad from the Texas Cardiac Arrhythmia Institute in Austin, US, explained that the treatment goal is electrical silence, to eliminate all electrical activity in the posterior wall, as part of the pulmonary vein (PV) ablation strategy.
Isolation is achieved in his practice at about 40 W, and typically with 5–10 g contact force (CF), but the catheter must continuously move and areas must be revisited. This approach to isolating the PV wall has been validated in meta-analyses for both paroxysmal and, even more so, persistent AF patients. Mitigating the risk to the oesophagus can be achieved by using a temperature probe that provides enough coverage of the oesophagus to be accurate,19 or even to move the oesophagus (although researchers are still trying to understand the safety and efficacy of this technique).
However, the question remains as to whether isolating the PV posterior wall is enough. Dr Al-Ahmad presented a case study that showed three of four PVs completely isolated after cryoablation. He asked attendees how they would manage the recurrence, and the audience was split between ablating the PV only, and ablating and looking for triggers.
“If you’re able to provoke arrhythmias, and you target those specific focal triggers, then you do indeed have some benefit in arrhythmia reduction. That’s been our strategy,” said Dr Al-Ahmad. “As we finish our AF ablation, we always look for PV triggers, and we document what they are. Now, if you take them for a third procedure, there are new PV triggers that are occurring – that tells us that this is a progressive disease. It makes sense. You can hit it for a while but ultimately new substrate does appear.”
In addition, pulmonary vein triggers are probably more important earlier in the disease progression and, as the disease progresses, non-PV triggers become more important. Techniques for eliciting them become the biggest challenge, along with how to target them for ablation successfully, efficaciously and safely.
Dr Al-Ahmad said the optimal extra-PV substrate ablation is complete isolation of the PVs, and his group includes the posterior wall of the left atrium. Then they identify triggers using high-dose isoproterenol and, if they can elicit triggers, they completely isolate the trigger substrate.