Sudden cardiac death (SCD) is common.1 It is responsible for half of cardiovascular deaths in developed nations and has been estimated to account for one in 10 of all deaths.1 A recent study estimated the annual incidences of SCD and out-of-hospital cardiac arrest in Europe to be approximately 249,500 and 343,500, respectively.2 Early defibrillation and advanced life support techniques allow successful resuscitation but, while survival rates are increasing, the overall outcomes of cardiac arrest remain poor.3
In resuscitated patients, sudden cardiac arrest (SCA) survivors, the priorities are to identify and manage the underlying cause and prevent or protect against future recurrence. The majority of SCA is the consequence of CHD, with approximately 80% due to either acute vessel occlusion leading to ischaemic VF or a pre-existing ischaemic scar providing a substrate for re-entrant ventricular tachycardia (VT).4 Furthermore, manifest structural or electric heart disease may already be evident on ECG and initial cardiac imaging with echocardiography.
However, no diagnosis will be apparent after initial assessment with ECG, echocardiography and coronary assessment in a significant proportion of SCA survivors. Such cases are referred to as unexplained cardiac arrest (UCA).5,6 Systematic evaluation of such patients may subsequently reveal a specific underlying diagnosis in nearly half of cases.7,8 Those in whom a diagnosis remains elusive receive the diagnostic label of idiopathic VF (IVF).
This review discusses the possible diagnoses in a survivor of an initially UCA and describes a systematic approach to investigation.
Prevalence of Unexplained Cardiac Arrest
The precise prevalence of UCA in the general population remains unclear. Historical estimates stated that 5% of cardiac arrest survivors had no evidence of ischaemic or structural heart disease, although a lack of accepted defining criteria was recognised.9 More recent prospective registry data reported that 12.3% of SCA survivors had no diagnosis after initial assessment with ECG, echocardiogram and coronary angiogram, indicating that the prevalence of UCA may have been underestimated in earlier studies.6 Of note, studies focusing on younger patients or those with SCA occurring in the context of exercise report higher proportions of UCA, so extrapolation of this figure to other populations may not be straightforward.10–11
Diagnostic Yield in Unexplained Cardiac Arrest
Several studies have reported the diagnostic yield of further evaluation of UCA cases, with variation largely depending on specific study inclusion criteria and populations studied.
The influential Cardiac Arrest Survivors with Preserved Ejection Fraction Registry (CASPER) is an on-going prospective registry of UCA cases from across Canada.5 A report of evaluation in the first 200 study participants identified a specific diagnosis in 41% of cases.7 Smaller European cohorts have reported yields of 50–61%.8,10 The national Cardiac Inherited Diseases Registry New Zealand reported an inherited condition diagnosed in 51% of 225 unrelated individuals whose first presentation was cardiac arrest.12
Differential Diagnoses of Unexplained Cardiac Arrest
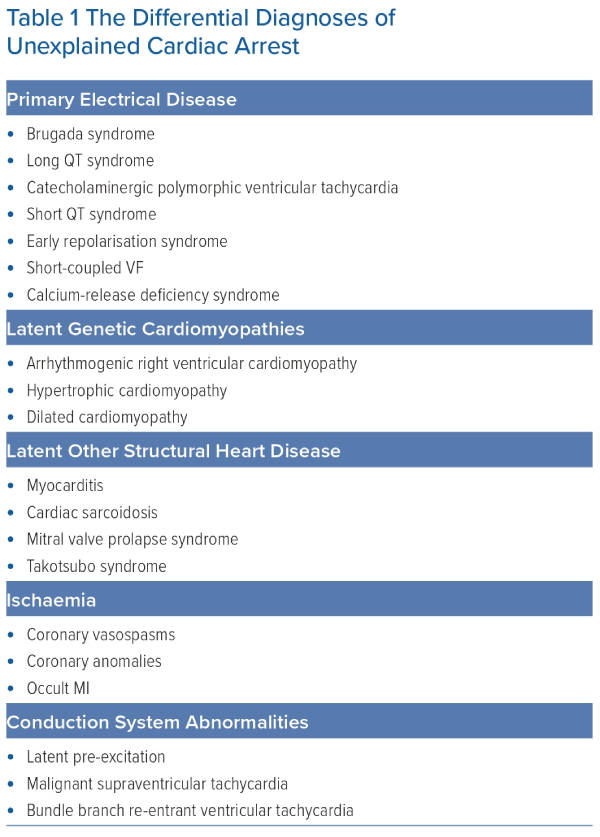
The differential diagnoses of UCA are broad (Table 1). Initially, non-cardiac causes of SCA, such as respiratory, metabolic or toxicological causes, should be excluded as far as possible. Thereafter, the differential diagnoses can be considered to include primary electrical disease or structural cardiac disease, which were not evident on original assessment.
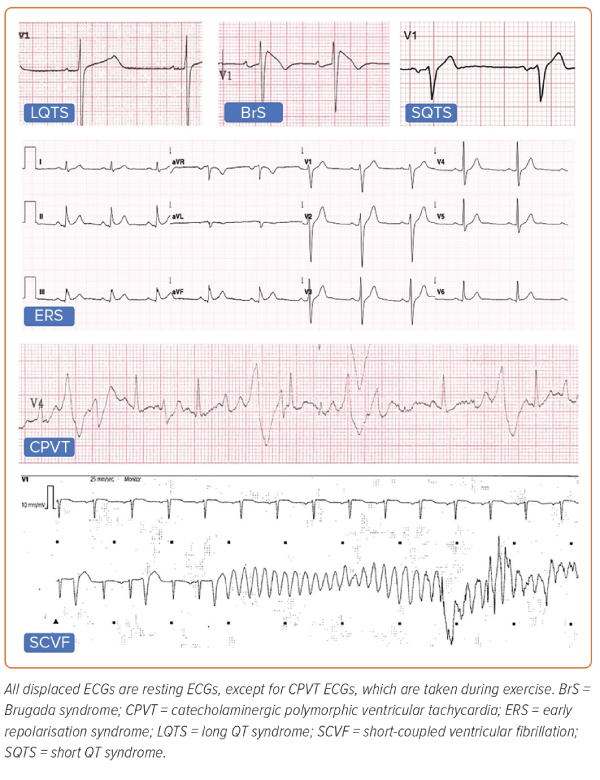
The established inherited primary electrical diseases (Figure 1) include Brugada syndrome (BrS), long QT syndrome (LQTS), short QT syndrome (SQTS), catecholaminergic polymorphic VT (CPVT) and early repolarisation syndrome (ERS).13 More recently described entities include calcium-release deficiency syndrome (CRDS), where episodes of polymorphic VT are caused by loss-of-function variants in the RYR2 gene, which encodes the cardiac ryanodine receptor 2.14 In contrast to CPVT, caused by gain-of-function variants in the same gene, arrhythmic episodes in CRDS are not triggered by exercise. Short-coupled VF (SCVF) is the preferred term to describe the long-recognised phenomenon of polymorphic VT or VF occurring in an otherwise normal heart repeatedly initiated by a short-coupled premature ventricular complex (PVC).15,16 There is now growing evidence that SCVF represents a distinct primary entity and efforts have been made to develop standardised diagnostic criteria.17,18
Structural heart disease, including concealed inherited cardiomyopathies, may also be diagnosed in UCA patients. Arrhythmogenic cardiomyopathy (ACM), including subtypes predominantly affecting the right ventricle (arrhythmogenic right ventricular cardiomyopathy; ARVC), left ventricle (LV) or both, can cause cardiac arrest, as can dilated cardiomyopathy (DCM) and hypertrophic cardiomyopathy (HCM). Additional structural causes of UCA include myocarditis and cardiac sarcoidosis.19 Takotsubo syndrome in its acute phase has been shown to be a cause for SCA.20 Mitral valve prolapse (MVP) has repeatedly been associated with a risk of SCA and should be considered as a potential diagnosis when identified in a UCA survivor. Associated findings of LV fibrosis, mitral annulus disjunction and LV ectopy, particularly in young women, support the diagnosis of the so-called arrhythmic MVP syndrome.21–23 The emerging importance of this syndrome as a cause for SCA is highlighted in a recent European Heart Rhythm Association expert consensus statement on arrhythmic MVP.24 Other reported diagnoses include latent ischaemia due to coronary vasospasm or malignant coronary anomalies, pre-excited AF due to latent pre-excitation, supraventricular tachycardias degenerating into VF and bundle branch re-entrant VT without evidence of structural heart disease.25–27
In cases where no diagnosis is made, the term IVF is used. Because IVF is a diagnosis of exclusion, its prevalence will depend on the diagnostic strategy used, as discussed below.
The Importance of Providing a Diagnosis
Making a specific diagnosis by thorough and systematic clinical testing following UCA is important for several reasons.28 Besides providing an explanation for the individual in an otherwise confusing and distressing situation, it leads to tailored treatment and prevention strategies not only for the individual, but also for family members, who may also be at risk for an inherited condition.
Although the vast majority of cardiac arrest survivors will undergo an ICD implantation for secondary prevention, there may be a minority – primarily CPVT patients – in whom the decision to implant an ICD is less straightforward.29
Similarly, cardiac arrest in the context of an acute myocarditis may not represent an increased long-term risk if myocardial function fully recovers and short-term protection with a wearable defibrillator may be sufficient.30,31 In the majority of cases where an ICD is indicated, the specific device type (e.g. transvenous versus subcutaneous ICD) and programming may be influenced by the diagnosis and relative likelihood of benefiting from bradycardia support, cardiac resynchronisation or anti-tachycardia pacing in the future.
In addition, appropriate medical therapy can be tailored to a specific diagnosis. Non-selective β-blockers are preferred in LQTS types 1 and 2 and flecainide may offer additional benefit in CPVT.32,33 In contrast, both offer little protection and may increase the risk of recurrence in BrS where quinidine may reduce the risk of further arrhythmic events.34,35 Quinidine also appears to be effective in SCVF.17 Diagnosis of cardiomyopathy may lead to consideration of treatments established as optimal medical therapy in heart failure.36
Several other medications may also precipitate arrhythmias or ECG changes in BrS or risk further QT prolongation in LQTS and are, therefore, recommended to be avoided. A comprehensive list for these drugs can be found online (BrS: www.brugadadrugs.org, LQTS: https://crediblemeds.org.37,38
Besides drug advice, specific diagnoses may also impact on other lifestyle advice, in particular related to physical activity and exercise. While exercise considerations apply to several inherited conditions, the specifics vary: patients with ARVC and CPVT may be counselled strongly against exercise.39 However, even in these two groups the exercise prescription may differ, with prolonged high-intensity exercise more detrimental in ARVC whereas CPVT patients must be aware that sudden high-energy exercises, or even other causes of adrenaline rush, such as playing video games, may lead to life-threatening arrhythmias. Within LQTS advice will vary depending on age, sex and underlying genotype with exercise, and swimming in particular, being an important trigger in LQT1. 40-43
Moreover, a specific diagnosis may guide early interventional therapy, as there is increasing evidence supporting early interventional approaches including catheter ablation for several inherited conditions. Ablation of areas of delayed depolarisation in the epicardial right ventricular outflow tract has been shown to reduce risk of future cardiac arrest in BrS.44,45 Ablation of VT/VF triggering His-Purkinje PVCs or even areas of subtle structural changes can also be considered in SCVF and ERS.46,47 Recent reports suggest early VT ablation in ARVC at the time of ICD implantation significantly reduced VT recurrence, driven by a reduction in ICD therapies.48 Left-sided cardiac sympathectomy is well established as an adjunctive treatment in LQTS and CPVT.49,50 Future prognosis and risk of recurrence can also be better informed by the specific diagnosis.
Finally, an early diagnosis is critical for the management of family members, as conditions leading to UCA are often heritable. Family screening is recommended to identify at-risk relatives and can be targeted with a specific diagnosis. In contrast, in IVF following comprehensive investigation of the index patient, family screening is unlikely to identify a clinically relevant heritable phenotype in first-degree relatives.51
Recommendations for Comprehensive Evaluation
For the above-mentioned reasons, a comprehensive work-up of sudden cardiac arrest survivors is critical, with particular focus on those cases that are initially unexplained, before assigning the label of IVF. Although there is consensus to further investigate UCA with extended diagnostic evaluation, the depth of these investigations has long been not clearly defined. Therefore, the extent of investigations markedly varied between centres and often remained at the discretion of the treating physician. However, the most recent European Society of Cardiology guidelines on the management of patients with ventricular arrhythmias and the prevention of SCD now propose uniform guidelines regarding the depth of these investigations in SCA survivors before the diagnosis of IVF can be made.52 The guidelines once again stress the importance of a multidisciplinary team effort overseeing the investigations. A multidisciplinary approach in optimising diagnosis of SCA aetiology during follow-up is also highlighted in a recent study improving the yield and time to diagnosis.53
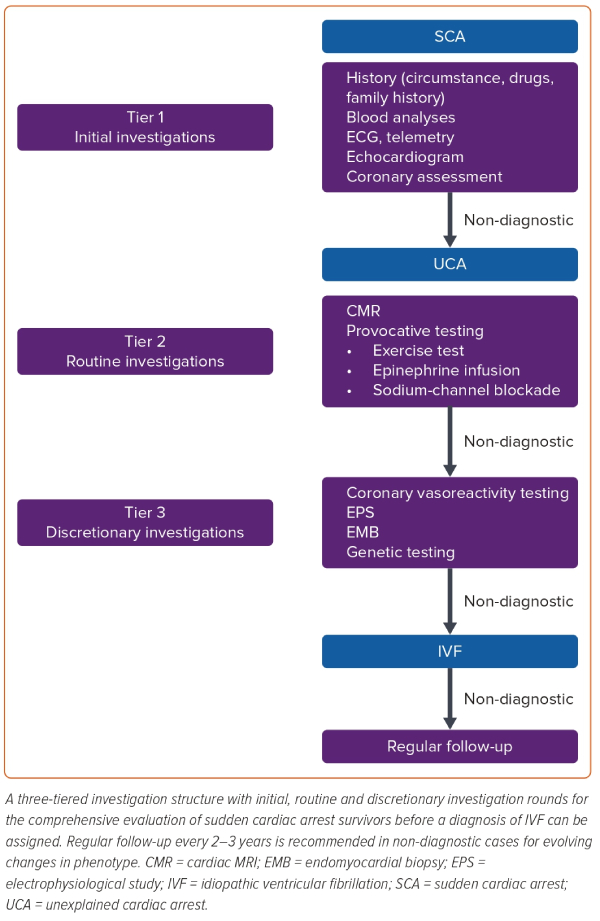
We describe a three-tiered investigation structure with initial, routine and discretionary investigation rounds to investigate SCA survivors (Figure 2). When a specific diagnosis is made, future management can be focused. However, it must also be noted that diagnostic confidence may vary and that understanding of both clinical features and underlying disease processes may evolve over time. Therefore, diagnoses and associated treatments may need periodical re-evaluation. This includes cases with no diagnosis after complete assessment as a more specific diagnosis may develop over time.54 In fact, SCVF is most often diagnosed at the time of a second arrhythmic event, when the short-coupled PVC triggering VF is documented on an ICD tracing.
Tier 1: Initial Investigations
Tier 1 investigations are aimed at the most common causes of cardiac arrest and should be performed as soon as possible after resuscitation. These investigations would be expected to find the cause in approximately 85% of resuscitated cardiac arrests.
The very early investigation of a cardiac arrest will overlap with the initial resuscitation and will particularly focus on potentially treatable/reversible causes. A history of the circumstances leading to the event can be extremely useful when available. Preceding chest pain raises the question of an acute coronary syndrome. Reports of recent drug use may suggest a toxic aetiology and concomitant illness such as profuse diarrhoea or vomiting may be indicative of electrolyte disturbance. Physical activity at the time of cardiac arrest can be suggestive of certain inherited conditions, such as ACM, HCM, CPVT or LQT1, but is non-specific. Once the patient is stabilised, a detailed history of preceding circumstances and prior medical history should be taken. Drug history should include prescribed and non-prescribed medications including over-the-counter medications, herbal remedies, supplements, and illicit drugs. As an initial screening for potential genetic causes, a three-generation family pedigree should be taken with a focus on previous SCD. Blood testing for electrolytes, cardiac enzymes and toxicology screening should be performed. Also at this early stage, suitable blood samples should be stored for potential future DNA analysis.
The first investigation available in the emergency setting will be the 12-lead ECG, performed to look for ST elevation suggestive of acute coronary syndrome with a view to proceeding to coronary angiography and primary percutaneous coronary intervention. Other ischaemic changes seen immediately following restoration of sinus rhythm from VF, such as T wave inversion and ST segment depression are non-specific and not necessarily associated with significant coronary artery disease. ECG findings associated with specific cardiomyopathies (e.g. HCM, ARVC) or primary electrical disease (e.g. type 1 Brugada ECG pattern, QT prolongation) may also be present. However, it must be noted that QT prolongation is frequently seen soon after resuscitation. Therefore, repeated ECGs following normalisation of non-specific post-arrest changes and correction of possible underlying electrolyte disturbances associated with resuscitation should be considered before a diagnosis of LQTS is made.
Coronary artery imaging is required given that the majority of cardiac arrests are due to ischaemic heart disease. This will allow exclusion of coronary artery disease, dissection, or coronary anomalies. In cases where the index of suspicion for coronary artery disease is low, a CT coronary angiogram can be used in lieu of an invasive angiogram. In patients with ischaemic ST elevation but unobstructed coronary arteries, coronary vasospasm should be considered. However, the logistic challenges of performing systematic provocation testing with ergonovine or acetylcholine in the post-arrest setting limit its use outside of cases with a high index of suspicion, perhaps contributing to the limited class 2b recommendation in current guidelines.52
Transthoracic echocardiography is used for an initial structural evaluation of the heart and to exclude cardiac tamponade. However, similar to ECG, initial echocardiography frequently shows non-specific LV dilation and reduced LV function soon after cardiac arrest as a consequence of myocardial stunning, which takes some days to improve. Therefore, caution must be exercised when diagnosing DCM soon after a cardiac arrest. However, echocardiography may already reveal diagnostic findings for overt cardiomyopathy such as asymmetric hypertrophy suggestive of HCM or right ventricular enlargement with regional dyskinesia as typically seen in ARVC. In addition, echocardiographic strain imaging is a sensitive measure of LV function and can detect subtle LV dysfunction, while LV ejection fraction is still preserved. Changes in specific strain measures have been shown to be associated with increased arrhythmic events.55,56 Echocardiography can also reveal the presence of MVP, perhaps with mitral annular disjunction, and raise suspicion for arrhythmic MVP syndrome. Equally, an apparently structurally normal heart will help to exclude certain causes, although the limited sensitivity of echocardiography to detect subtle fibrosis should be considered. Where tier 1 investigations are non-diagnostic, the term UCA is used and investigation should proceed to tier 2.
Tier 2: Routine Investigations
In UCA, tier 2 investigations focus on revealing either subtle cardiomyopathy or latent primary electrical disease. It is the opinion of the authors that all tier 2 investigations should be undertaken before IVF can be diagnosed. Recent algorithms for UCA evaluation to assess the strengths of IVF diagnosis also support this approach for a definite IVF diagnosis.57
Cardiac MRI
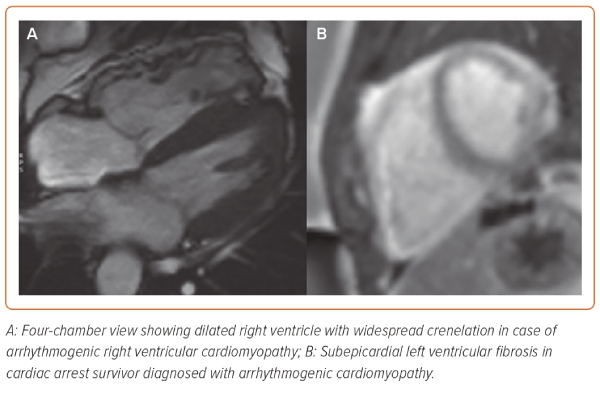
Cardiac MRI (CMR) has emerged as the imaging modality of choice for the diagnostic evaluation of UCA because of its ability to identify more subtle disease at an early stage.58,59 CMR is therefore suitable for the diagnosis of concealed cardiomyopathy, takotsubo syndrome, inflammatory conditions, such as acute myocarditis or cardiac sarcoidosis (CS) and infiltrative diseases (Figure 3). In addition to accurate and reproducible chamber quantification and ejection fraction measures, its added diagnostic value comes with the tissue characterisation techniques of CMR. Late gadolinium enhancement (LGE) and other quantitative parameters, such as T1-mapping allow the identification of fibrosis, while T2-weighted sequences detect myocardial oedema.60 In the context of UCA, the combination of these sequences and the anatomical distribution of abnormalities can potentially distinguish between an acute and likely reversible lesion and a chronic and irreversible one.59 Detection of oedema is reflective of inflammatory conditions, such as myocarditis; however, sub-endocardial distribution of oedema is associated with myocardial ischaemia. The presence and pattern of LGE in the myocardium can differentiate between various cardiomyopathies but can also be diagnostic for chronic infiltrative conditions, such as CS. Recent evidence supports that, within ACM, the distribution of LGE differs between underlying genotypes and is a principle driver of the potential reclassification of these conditions depending on genotype.61,62 Finally, the presence and extent of LGE may predict the risk of recurrent arrhythmic events.63
Provocative Testing
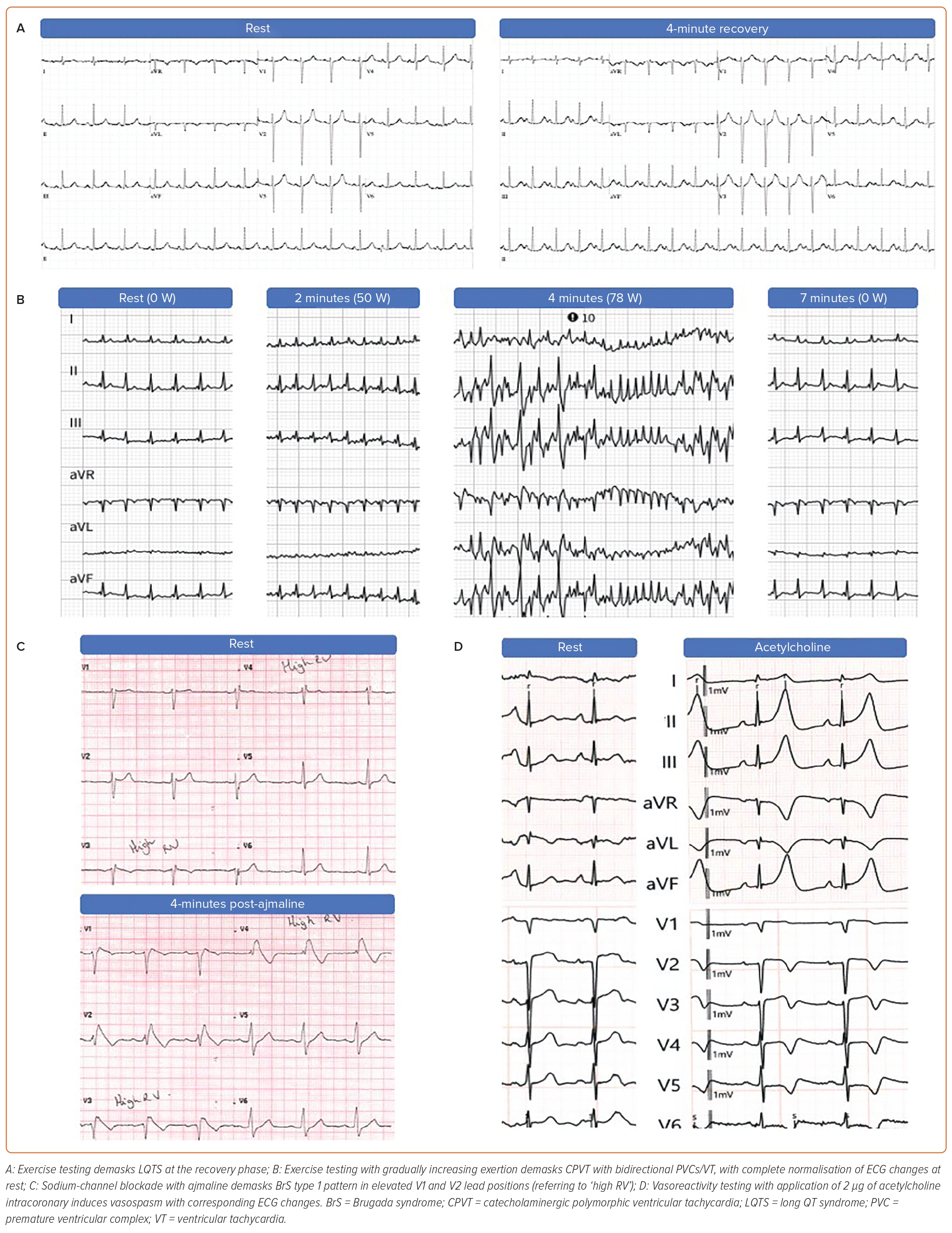
The pathognomic ECG changes seen in the most commonly diagnosed primary electrical diseases, LQTS and BrS, can often be transient, but may be unmasked by provocative testing (Figure 4). Furthermore, CPVT patients will have a normal resting ECG with abnormalities typically only seen after exercise provocation.
Provocative Testing for Long QT Syndrome
The QT interval is known to be variable throughout the day, influenced by heart rate and reflected in changes of autonomic tone. In LQTS patients, a maladaptive repolarisation response is frequently seen so that the usual shortening of repolarisation times at higher heart rates is not possible and paradoxical QT prolongation is seen.64 Two provocative tests have been developed to assess the repolarisation reserve, namely exercise testing and pharmacological provocation with isoprenaline infusion. Historically there has been much interest in the use of exercise testing to diagnose LQTS, although initial efforts were limited by difficulties in precise measurement of the T wave at rapid heart rates and by the significant artefact caused by exercise. More recently, it was found that QTc prolongation in the recovery phase after exercise can be used to accurately diagnose LQTS in suspected cases with a normal or borderline QT interval at rest.65 Specifically, a QTc ≥480 ms at the fourth minute of recovery post-exercise most clearly differentiates affected from unaffected individuals and is included as a diagnostic feature in LQTS scoring systems Figure 4A.66
Epinephrine infusion is an alternative provocative test to stress QTc interval for unmasking LQTS. Early studies with epinephrine infusion demonstrated typical paradoxical QT prolongation, which was able to differentiate patients with LQT1 from unaffected relatives.67,68 However, the test performed less well when used in the CASPER trial. An abnormal result was reported in 18% of SCA survivors, with a borderline result in a further 14%.69 However, there was only modest correlation between abnormal response to epinephrine and exercise and only 17% of individuals with abnormal epinephrine response were genotype-positive for LQTS. The authors therefore concluded that epinephrine testing is likely to be sensitive for LQTS, but the specificity is questionable.69 We therefore recommend exercise testing as the preferred provocative test for the evaluation of LQTS with epinephrine not routine used.
Provocative Testing for Catecholaminergic Polymorphic Ventricular Tachycardia
Unlike the other inherited arrhythmia syndromes, CPVT is characterised by a normal baseline ECG with induction of arrhythmia by adrenergic stimulation. Therefore, provocative testing with exercise or epinephrine infusion is crucial for the diagnosis of this condition (Figure 4B). The pathognomonic feature is bidirectional or polymorphic VT during exercise or epinephrine infusion, although isolated ventricular ectopics or ventricular bigeminy may be the only positive finding in some cases.
Studies using similar epinephrine infusion protocols to those for LQTS have demonstrated variable sensitivity with controls rarely demonstrating abnormal results.69,70 However, the lack of a gold-standard test or large systematic studies means that, as for LQTS, the true specificity remains unclear and exercise testing is preferred.
Provocative Testing for Brugada Syndrome
Only the type 1 Brugada ECG pattern (‘coved-type’) is considered diagnostic of BrS.34 It may be present spontaneously following resuscitation, although, more commonly, other related ECG changes will raise suspicion of the diagnosis including type 2 or 3 Brugada ECG patterns. However, the latter are not diagnostic of BrS and provocation testing is required to unmask a type 1 BrS pattern.71 Since the ECG changes seen in BrS correlate with abnormalities of the right ventricular outflow tract, which is commonly positioned superior to the standard V1 and V2 ECG electrode positions, modified ECGs with high right ventricular leads (in the second and/or third intercostal space) are recommended as they have been shown to increase sensitivity of observing a type 1 Brugada ECG pattern.72–76
In cases with non-diagnostic ECGs (e.g. type 2/3 patterns), particularly if the clinical circumstances of the cardiac arrest are suggestive of BrS (nocturnal, precipitated by fever), sodium channel blocker (SCB) testing is recommended to unmask the type 1 Brugada pattern (Figure 4C). Ajmaline, flecainide, procainamide or pilsicainide can be used. Ajmaline has the benefit of a very short half-life, minimising the length of the test and the need for prolonged monitoring afterwards and is most commonly used in Europe. The recommended dose is 1 mg/kg given intravenously over 10 minutes, with false-positive results occurring more commonly at higher doses.34 Precipitation of a type 1 Brugada ECG pattern in one or more leads constitutes a positive result.13 However, poor specificity of SCB provocation remains a concern and may also vary depending on the drug used.77 Therefore the current task force consensus as well as the recent VT guidelines require other clinical features, such as documented PVT/VF, arrhythmic syncope or relevant family history in case of an induced type 1 ECG pattern for the diagnosis of BrS.34,52 While a positive test in patients resuscitated from UCA meets diagnostic criteria as per recent guidelines, it is recommended that other tier 2 investigations are performed before SCB provocation and thought is taken where other clinical findings may be incompatible with BrS.
Tier 3: Discretionary Investigations
If a specific diagnosis remains elusive upon completion of tier 2 investigations, further diagnostic evaluation should be considered on an individual basis regarding clinical suspicion for specific conditions. These tests might include coronary vasoreactivity testing, electrophysiological study (EPS), endomyocardial biopsy, further imaging studies or genetic testing.2
Provocative testing for coronary vasospasms can be performed if suspicion of a coronary ischaemia-related SCA remains, especially if CMR findings suggestive for an occult MI or symptomatic transient ST elevations were documented during continuous heart rhythm monitoring. The most established approach for vasoreactivity testing is by intracoronary infusion of acetylcholine, which triggers vasoconstriction via muscarinic receptors on endothelial and vascular smooth muscle cells (Figure 4D).78 The Paris Sudden Death Expertise Center study, a prospective, population-based registry in the greater Paris area, which systematically investigates every case of adult out-of-hospital SCA, found that 2% of SCAs of cardiac cause were related to coronary vasospasm.79 In this cohort, provocative testing for coronary vasospasms was performed in 63.9% of UCA patients with a diagnostic yield of 30.8%. The authors concluded that the burden of vasospasm-related SCA is likely underestimated because of the lack of systematic testing in UCA survivors, although further research is needed to evaluate the specificity of such testing in UCA.
Although EPS with programmed electrical stimulation is useful in evaluating inducibility of VT in patients at risk, currently it is not routinely recommended in the work up of UCA as its incremental diagnostic value is considered to be low based on earlier studies.52 Pre-excited AF with latent accessory pathway and only subtle pre-excitation on a resting ECG or other supraventricular tachycardias degenerating into VF are recognised causes of cardiac arrest. Wang et al. reported the results of 290 consecutive EPS in cardiac arrest survivors which identified a supraventricular tachycardia as the cause in 4.5%.26 Atrio-ventricular re-entrant tachycardia due to an accessory pathway was seen in six of 13 positive cases, one of whom also had HCM. Patients were studied from 1979–1990 and wider changes in the diagnostic approach to cardiac arrest since this time should be considered. Nevertheless, EPS is still justified on an individual basis depending on the pretest probability.80 Further, EPS may reveal bundle branch re-entrant VT, without evidence of structural heart disease, as a cause for resuscitated cardiac arrest.27 In case of suspicion for these conditions, EPS is indicated, given the possibility of a curative treatment for these conditions thereby preventing ICD implantation and its associated long-term complications. Further clarification on the yield of standardised EPS in UCA is expected from the on-going prospective observational EPS ARREST study (NCT03079414).
In addition, for suspected occult ARVC, voltage mapping can be performed to assess for scar and inducible VT substrate.81 Similarly, recently it was shown that endocardial and epicardial high-density electrophysiological mapping was able to identify subclinical microstructural cardiomyopathic areas in a subset of IVF patients acting as substrate for VF re-entries.46,82 Besides revealing a diagnosis, their precise phenotypic characterisation would also have implications for individualised therapy by means of ablation therapies. In addition, as shown recently, EPS can be used as a promising diagnostic test for the newly described channelopathy CRDS.83 Hereby a specific stimulation protocol consisting of a long burst, long pause and short-coupled ventricular extrastimuli is used for the induction of arrhythmia. Because the variable inducibility of arrhythmia correlated with the arrhythmia burden of the patients, it was suggested that this EPS protocol might also be useful for risk stratification in these patients.
Endomyocardial biopsy (EMB) is only rarely required for the work up of UCA. Although EMB is considered the gold standard for the diagnosis of CS, as a positive EMB definitely establishes the diagnosis of CS, the sensitivity of this invasive approach is low because of the patchy involvement of the disease.84 In addition, non-invasive imaging studies such as CMR or 18F-fluorodeoxyglucose PET have become the preferred tools for the diagnosis of CS.
Genetic Testing
Because many causes of an initially UCA may be heritable, there is a clear role for genetic testing in a range of scenarios following UCA. Definite diagnosis of a heritable condition should lead to genetic testing specific to that condition, with testing focused on genes with robust evidence for causation. The expected likelihood of identifying a pathogenic or likely pathogenic variant varies between conditions, e.g. from approximately 20% in BrS up to approximately 80% in LQTS.28,52 Since the yield does not reach 100% for any inherited condition, negative genetic testing in the index patient does not exclude a genetic aetiology and family members may require clinical screening regardless of the genetic testing results when confidence in the proband’s diagnosis is high.
The role of genetic testing in cardiac survivors where clinical investigation does not reveal a diagnosis (i.e. IVF) is less clear. Retrospective analyses have shown diagnostic yields of up to 17% in UCA populations and approximately 10% in those where in-depth clinical assessment has been performed.85,86 Of note, variants identified in these populations have frequently been in genes associated with cardiomyopathy rather than primary electrical disease. This raises the question of how these genetic variants are causally linked to the cardiac arrest. Hypotheses include that a hitherto concealed cardiomyopathic process presents first with ventricular arrhythmia or that the molecular changes directly alter intracellular electrophysiology and decrease the fibrillation threshold. Observations that similar genes are implicated in young-onset AF suggests these variants are the ‘first hit’ that increases an individual’s susceptibility to arrhythmia when exposed to as yet unidentified environmental factors.87,88
Long-term Follow-up
Long-term follow-up of patients with initially UCA is recommended as arrhythmic recurrence is frequent and diagnostic classification may evolve. Observational cohort studies have reported recurrent arrhythmia in 16–26% of patients.7,89–91 Events are most common in the first few years after presentation, with a median time to shock of 1 year (interquartile range 1–3 years) and more common in those patients without comprehensive evaluation at first assessment.91
Merghani et al. retrospectively analysed 360 cardiac arrest cases and found that the diagnosis changed in a significant number.53 Of the 45 patients with a structurally normal heart, the proportion with a specific diagnosis rose from 31% to 64% over the follow-up period. The multicentre Dutch iVF-Registry reported that 9% of patients originally labelled as IVF received an alternative diagnosis over a median follow-up of 6 years. 2–12,91 Patients with more complete evaluation at baseline were less likely to receive an alternative diagnosis, reflecting the importance of the systematic approach described above. However, even patients with idiopathic VF may develop a more specific cardiac phenotype over time.
Conclusion
The cause of a cardiac arrest due to VT/VF remains unexplained in a significant minority of patients after initial evaluation with ECG, echocardiogram and coronary imaging. A systematic approach to further assessment is recommended to maximise the chance of making a specific diagnosis. Inherited primary electrical disease and cardiomyopathies are often identified as the aetiology in these cases. Establishing a diagnosis of these heritable conditions is not only relevant for the management of the index patient but also their family members at risk for the same condition. Given the complexity and rare nature of these conditions involving the care of entire families, patients should be managed in centres with multidisciplinary teams including expertise in cardiac electrophysiology, imaging and cardiogenetics, as emphasised in the current expert consensus.28 Although we have come a long way in revealing the aetiology of UCA with refined diagnostic tools, further research is needed to ultimately explain the unexplained. 
Clinical Perspective
- The cause of cardiac arrest is initially unknown in a significant minority of cases.
- Systematic investigation of such unexplained cardiac arrests will provide a specific diagnosis in up to half of individuals.
- A systematic, tiered approach to investigation is suggested.