Anatomically based pulmonary vein isolation (PVI) is the cornerstone of ablation, yet continues to achieve success rates of 55–75% at 12–18 months in randomised trials, depending on the population, which falls over time.1 Notably, there has been little benefit from adding several anatomical ablation strategies targeting the posterior wall, the mitral isthmus, left atrial roof or other linear lesions.2–5 Studies that add isolation of the left atrial appendage are ongoing.6 A dominant alternative viewpoint is that patients with AF likely differ in the anatomical locations for their mechanisms, underscoring the need to optimise AF mapping. This viewpoint is tempered by the reality that mapping systems to date have yielded divergent data, varying outcomes in different clinical series and complex mathematical approaches that are difficult to validate by clinical observation.7 In contrast, PVI and anatomical ablation lines are relatively straightforward to confirm clinically.
This review critically evaluates the bench-to-bedside evidence that localised regions of interest perpetuate AF, and describes clinical approaches used to identify these regions for ablation.8,9 We will attempt to address why the localised source hypothesis may be more important to some patients than others.10–15 We will intersperse our descriptions with thoughts on future directions to address challenges that we believe must be overcome to truly advance the field.
Mechanisms that Sustain Human AF
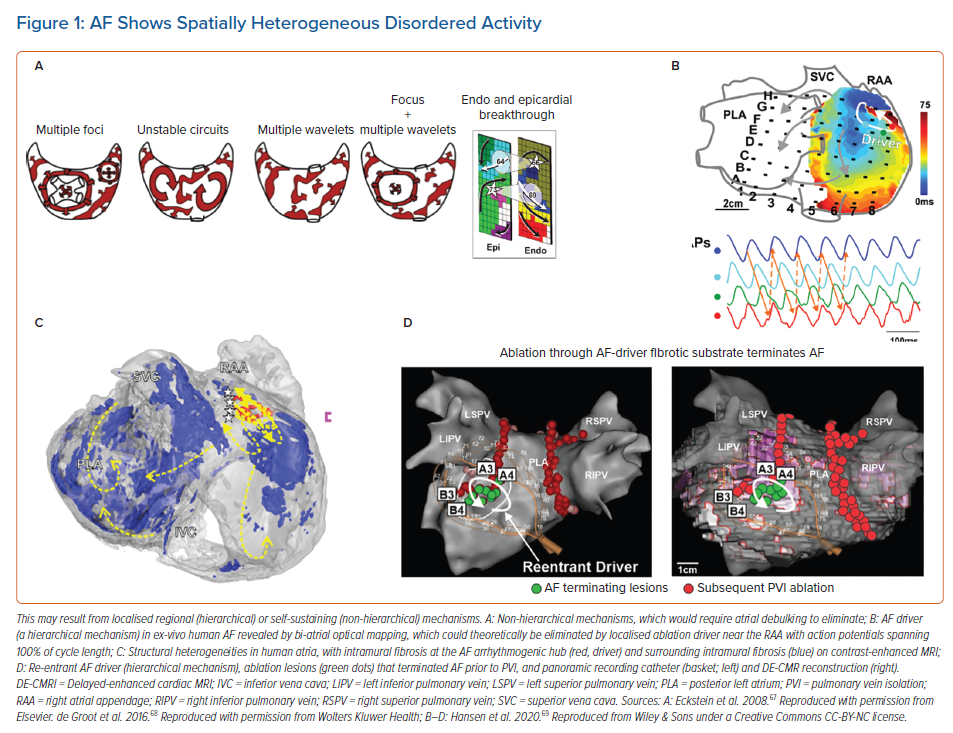
Once AF has been initiated by triggers from the pulmonary veins or other sites, its disorganised wavefronts must be continuously replenished for AF to sustain.16 Two mechanisms are proposed. In one model, disorganised activity self-sustains because new wavelets are generated across atrial tissue over time. Computer simulations reveal that such wavelets can be generated by unstable spiral waves and wavebreak.17,18 Collision from repetitive foci in multiple spatial locations can also destabilise wave propagation, again producing new wavelets.19 This hypothesis does not require preferred regions of interest (Figure 1A), and effective therapy would require large-scale debulking of the atrium. In the second major model, preferred regions of interest are central to perpetuating AF. Such regions may represent localised rotational or focal sources, other electrical features such as repetitive activation patterns, or structural regions of fibrosis or tissue anisotropy that replenish disordered wavefronts and can thus be termed ‘drivers’ (Figures 1B, 1C and 1D).
Localised rotational or focal sources are a well-described mechanism for replenishing AF wavelets. Rotational circuits were first described in animal models of ventricular and AF in seminal work by Jalife and others in the 1990s.9 Classically, a rotor is defined as re-entry around an unexcited yet excitable core, activating too rapidly for surrounding tissue to keep up and resulting in disordered waves (‘fibrillatory conduction’). This has been shown in multiple animal studies using optical mapping and contact mapping and more recently in human atria during AF (Figures 1B and 1C).9 These features have been reported in patients by many techniques, and ablation at these localised sites alone may terminate AF (Figure 1D).8,20 The field has been confused by argument on the definitions of a rotor, yet this argument has little practical significance at the spatial resolution of clinical mapping. We use the terms rotational activation, localised re-entry and rotor interchangeably, leaving ultimate adjudication on this issue to future biophysical, tissue-level and cellular studies. Rapid focal activity can also produce fibrillatory activity and may be a source or driver for AF. The localised source hypothesis may explain how AF sustains in small volumes such as mouse hearts.21 This has always been difficult to explain by the multiwavelet hypothesis that requires large volumes, and how limited ablation alone may terminate persistent AF, which is also difficult to explain by the multiwavelet hypothesis.7,22
Clinical Mapping of AF in Patients
An increasing variety of approaches are available to map AF. Despite differences in how signals are recorded and analysed, most clinical systems reveal organised features in AF in both atria where ablation can acutely impact AF in patient subsets.
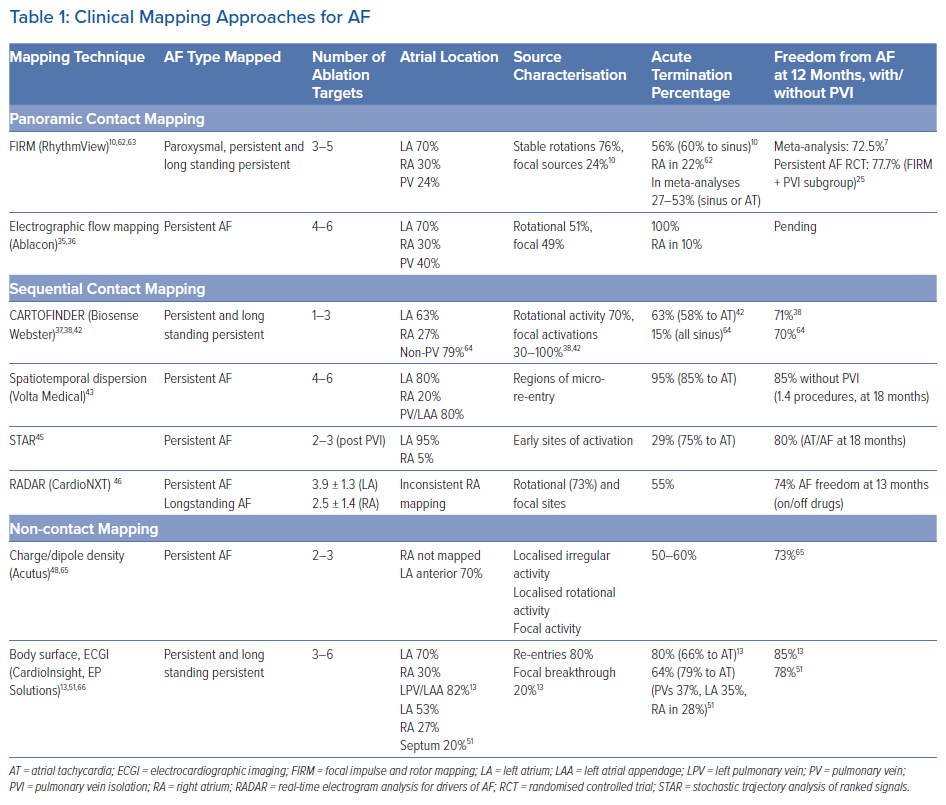
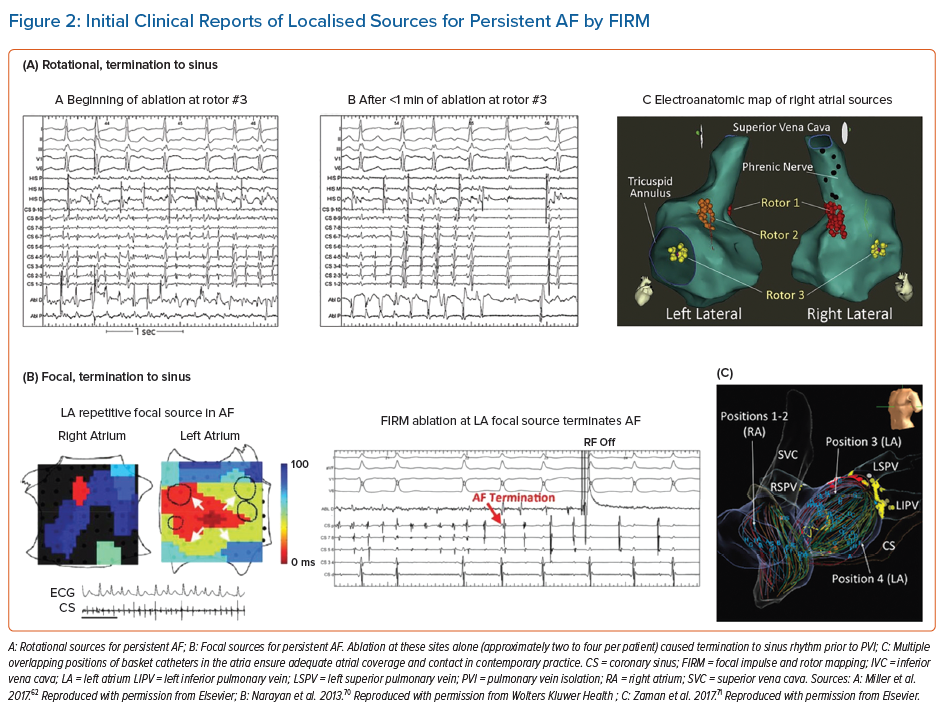
Table 1 summarises AF clinical mapping systems. Essentially all identify localised regions of interest in AF, such as focal impulses, rotational sources or other repetitive areas (including ‘localised disorder’). Spatially, three to five such sites are typically seen in each patient, approximately two-thirds of which are in the left atrium. Figures 2A, 2B and 2C show examples of mapped sites where ablation terminates AF. Multiple sites often co-exist and compete and, as shown in Figures 2D and 2E, when the re-entrant site (blue) dominates AF the focal site (pink) is quiescent and vice versa.23 The earliest clinical mapping system (focal impulse and rotor mapping [FIRM]), specifically designed to identify focal sources, provides ~80% concordance with simultaneous optical mapping of human AF, with the caveat that electromechanical decoupling agents were used to eliminate atrial motion (a general requirement for optical mapping).24 Validation studies of other clinical mapping systems are pending.
We summarise reported AF mapping methods based on whether their primary recording approach is global (panoramic) or in a small field of view (and hence sequential) in the atria. Sequential approaches assume that AF sources, when identified, are relatively stationary during the timescale of mapping or return to preferred locations repetitively if they meander. We also compare systems based on whether they use contact electrodes, which have traditionally been the gold standard, or non-contact recordings such as body surface mapping, in which electrograms are computed mathematically. Randomised trial data do not yet support the benefits of ablating targets identified by any of these mapping systems, but early subset analyses of randomised trials are promising and several trials are ongoing (NCT04442113, NCT04473963, NCT02696265, NCT04428944, NCT04702451).25
Panoramic Contact Mapping
Focal Impulse and Rotor Mapping
FIRM was the first approach to systematically map localised putative drivers for AF and target them for ablation (Figure 2). FIRM analyses unipolar electrograms of AF from wide-area (panoramic) basket recordings, and applies physiological filters based on monophasic action potential dynamics and conduction velocity to remove noise (RhythmView, Abbott). Initial reports of FIRM in 2011 demonstrated localised rotors and focal sources in nearly all patients with AF, where ablation was able to terminate or slow AF and eliminate AF in 82.4% of patients. Results from this mapping approach are ~80% concordant with concurrent optical mapping of AF in explanted human atria, with promising results of ablation in meta-analyses.7,24,26,27 However, some clinical studies reported very poor results, for unclear reasons. Nevertheless, this has motivated newer techniques to overcome difficulties in reading maps, to provide better atrial coverage during AF, to better define ablation strategies once mapping is complete and also to identify patients in whom these mechanisms are more important than others.
AF sources from FIRM mapping arise in diverse locations; one-third in the right atrium.11,28,29 This intriguing figure may explain the 70–80% success ceiling of left atrial ablation for AF and the reported benefits of right atrial ablation in some patients.30,31 There were few complications during mapping.32 In a recent randomised clinical trial (REAFFIRM), intention-to-treat analysis showed no difference in arrhythmia freedom between FIRM + PVI and PVI. However, in on-treatment analysis, the pre-specified PVI + FIRM group showed beneficial trends over the prespecified PVI alone group (77.7% versus 65.5% single procedure freedom from atrial arrhythmias at 1 year; p=0.09).25 These hypothesis-generating results are now being tested in on-going randomised trials of several AF map-guided ablation strategies.
FIRM studies in 2011–2014 foreshadowed several current controversies in AF mapping. First, AF driver sites fluctuated over time, but some reappeared in conserved locations for very prolonged periods.33 While reappearing features were ideally prioritised for ablation, they may have been difficult to identify at some centres. A major focus is to reduce subjectivity in map reading, for which machine learning has had some success.34 AF sources may fluctuate if they compete with concurrent sites.23 From optical maps of human AF, fluctuations may also represent intramural migration of a driver.8 Conversely, body surface mapping (electrocardiographic imaging; ECGI) interprets these features as truly intermittent.13
Electrographic Flow Mapping
Electrographic flow (EGF) uses panoramic basket catheter recordings to compute the ‘average propagation of action potentials.’35 Unipolar electrogram data are used to reconstruct 64 discrete data streams with equal peak amplitudes. After filtering to reconstruct local extracellular voltage, EGF reconstructs an electrogram that is stated to be akin to an optical action potential at each electrode. One hundred consecutive voltage shapes covering 1.9 seconds are then fed into a Horn-Schunck optical flow algorithm to calculate average flow behaviour. Because of noise and imperfect mathematical modelling, flow vectors may scatter widely with random vector sizes and directions. Averaging cycles eliminates these random vectorial components, leaving consistent EGF vectors that dominate the final map.
EGF vectors may be consistent for several reasons. Flow vectors are constant at a given point if potentials originate from a local source, in which case they may travel in circular or spiral re-entry pathways, flow around a fixed anatomical obstacle, or follow a linear pathway. This principle of vector summation is a critical stated advantage over phase or activation mapping, where noise and artefacts cannot be easily eliminated by temporal averaging. The entirety of the resulting constant vector components is comprised in flow maps.
This approach has been proposed to distinguish primary (true) from secondary (passive) regions, although this has yet to be verified. Applied retrospectively to FIRM data, the approach identifies similar regions to FIRM including at sites where targeted ablation terminated AF.36 In that study, sites labelled as ‘active’ versus ‘passive’ did not identify sites of termination and non-termination. The commercially available system, Ablacon (Ablamap, Ablacon Inc.) is currently undergoing prospective evaluation to identify areas of importance for AF ablation.
Sequential High-density Contact Mapping
CARTOFINDER
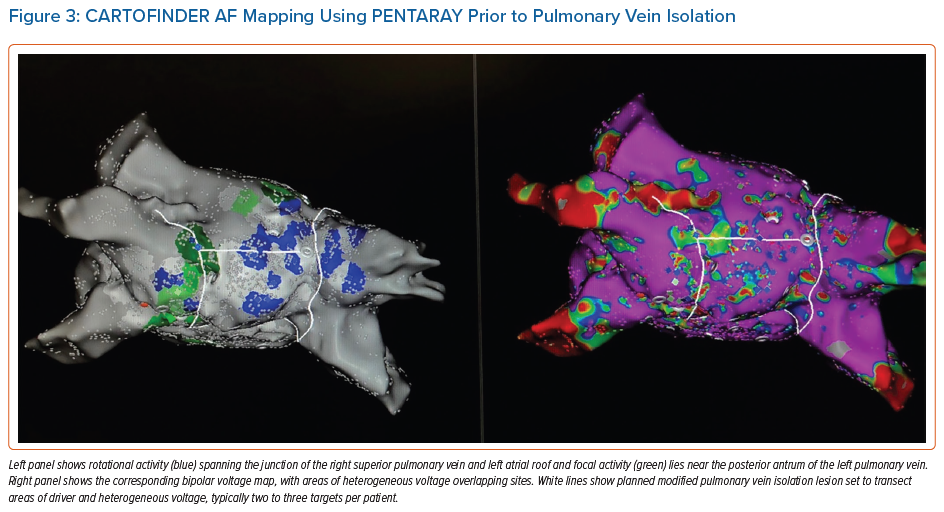
CARTOFINDER is the system from Biosense Webster integrated into current versions of the CARTO electroanatomic mapping system. CARTOFINDER uses combined unipolar and bipolar electrogram annotation to construct high density activation maps using either a panoramic basket catheter (Constellation, Boston Scientific) in early studies, or a high-density PENTARAY (Biosense Webster) in more recent series.37–40
Mapping using CARTOFINDER can be integrated into routine clinical workflows, as it requires no additional equipment if one is already using CARTO for AF ablation if the software module is present.41 Mapping shows focal and rotational activation patterns as regions of interest (ROI).
Figure 3 shows a case of a 47-year-old man with new onset de novo persistent AF with extensive areas identified using CARTOFINDER pre-PVI, which were incorporated into the lesion set and ablated in addition after completion of PVI. Repeat mapping was performed to ensure modification of the ROI as an endpoint.
In recent series, there does not appear to be a relationship between these sites and low bipolar voltage as measured by the PENTARAY, in contrast to earlier work using basket catheters.41,42 Given the large number of institutions that use CARTO mapping as part of an AF ablation, more systematically collected data are emerging to guide mechanistic insight and tailored therapy approaches using this method.
Spatiotemporal Dispersion Mapping
In 2017 Seitz et al. described the use of spatiotemporal dispersion to identify areas of stable electrogram patterns across the splines of a high density PENTARAY catheter, which spanned the AF cycle length and on modelling were proposed to represent electrogram fingerprints of nearby rotational drivers.43 Targeting these areas, without the need for activation assignment or annotation of electrograms, enabled high acute termination rates and high freedom from AF in a single-arm trial versus historical results from PVI only. Notably, the authors did not perform PVI in this series, with only spatiotemporal dispersion areas being targeted for ablation, but subsequent series have found it additive to PVI in terms of clinical efficacy.43,44
This approach has now been automated using artificial intelligence classification tools in a software system called Volta (Volta Medical), compatible with any multipolar catheter, currently under investigation in the Ev-AIFIB trial (NCT03434964) at eight sites in France.
Stochastic Trajectory Analysis of Ranked Signals
Stochastic trajectory analysis of ranked signals (STAR) mapping identifies atrial regions in AF that most often precede activation of neighbouring areas, calculated from multiple individual wavefront trajectories. By gathering data from hundreds of activations, a statistical model is formed, permitting regions of the atrium to be ranked by the amount of time that their activations precede those of adjacent regions.
The method imports electrogram signals, corresponding chamber geometry and catheter location data from electro-anatomical mapping systems. STAR maps allow the earliest sites of activity (ESA) to be identified on a digital replica of atrial geometry. For a site to be classified as an ESA it must lead for >75% of the time. STAR has been applied to panoramic contact baskets and as small multipolar mapping catheters. One ‘advantage’ of the approach is that it does not discriminate whether a site is rotational, focal or shows other activation patterns. The ablation strategy of targeting of areas of ESA is the same for each. In a recent single-centre study of 35 patients, after PVI an average of 2.6 ± 0.8 ESA were ablated. Out of the 86 STAR maps created post-PVI, the same ESA was identified on 73.8 ± 26.1% of maps. ESAs that resulted in AF termination were more likely to be identified on both pre- and post-PVI maps. During a follow-up of 18.5 ± 3.7 months, 28 (80%) patients were free from atrial tachycardia/AF.45 Additional data on this technique are pending.
Real-time Electrogram Analysis for Drivers of AF
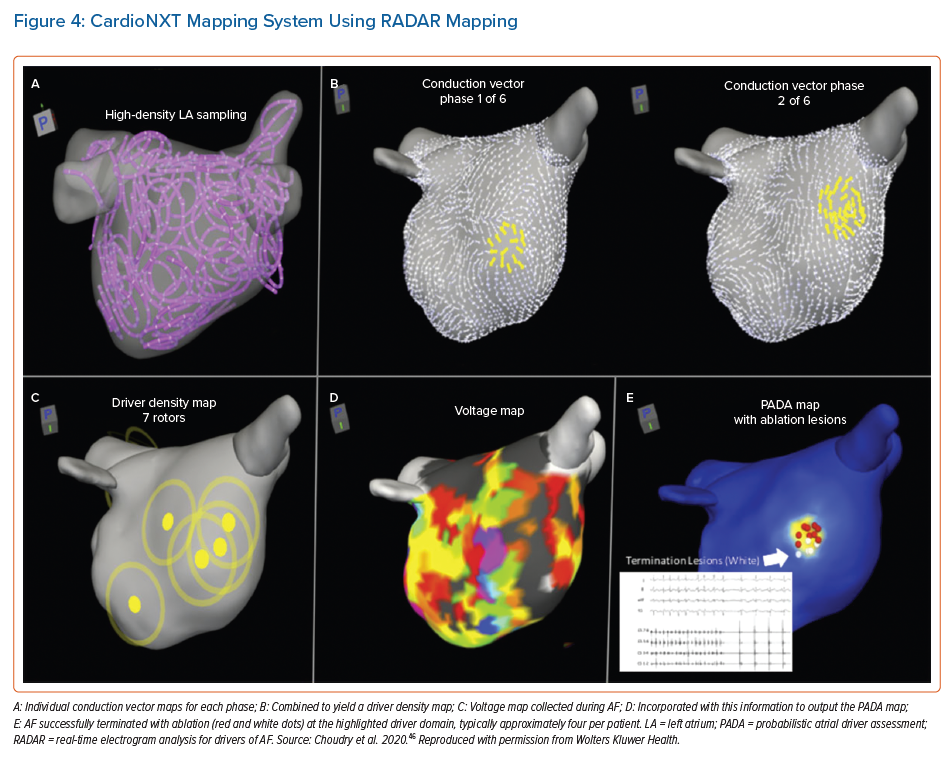
Another sequential high density mapping method for AF is termed real-time electrogram analysis for drivers of AF (RADAR). Using the coronary sinus as a reference, this system sorts and compiles electrograms from various anatomical locations to create a panoramic 3D conduction vector map that corresponds to each of a series of numerically calculated ‘phases’ or patterns of coronary sinus activation. Distance between electrodes is calculated using positions obtained from the electroanatomical mapping system. Using these distances and depolarisation timings at each electrode, conduction vector velocities are calculated between electrode pairs. An average of 587 electrode locations are used to map the chamber. Conduction vectors are computed using combinations of all of these electrode positions and depolarisation timings.
The commercial system (CardioNXT, CardioNXT.) computes sites of rotational activity and focal impulses from individual conduction vector maps, and combines them to create a driver density map (Figure 4). When rotational or focal activity is detected in voltage border zones, the region is highlighted. These data are fused to create a single probabilistic atrial driver assessment map that incorporates repetition of drivers and areas of voltage transition to highlight putative AF driver domains, which can then be targeted for ablation.46
Initial results from ablation using this approach show 68% off-drug freedom from AF and 74% when on/off drug after a 13-month follow-up in 64 patients treated.
Non-contact Charge Density Mapping
Non-contact charge density mapping is an alternative approach to map non-stationary AF mechanisms, inspired by the non-contact technology used to map focal ventricular ectopics and appreciation of the membrane-associated charge origin of the cardiac electrical field.47
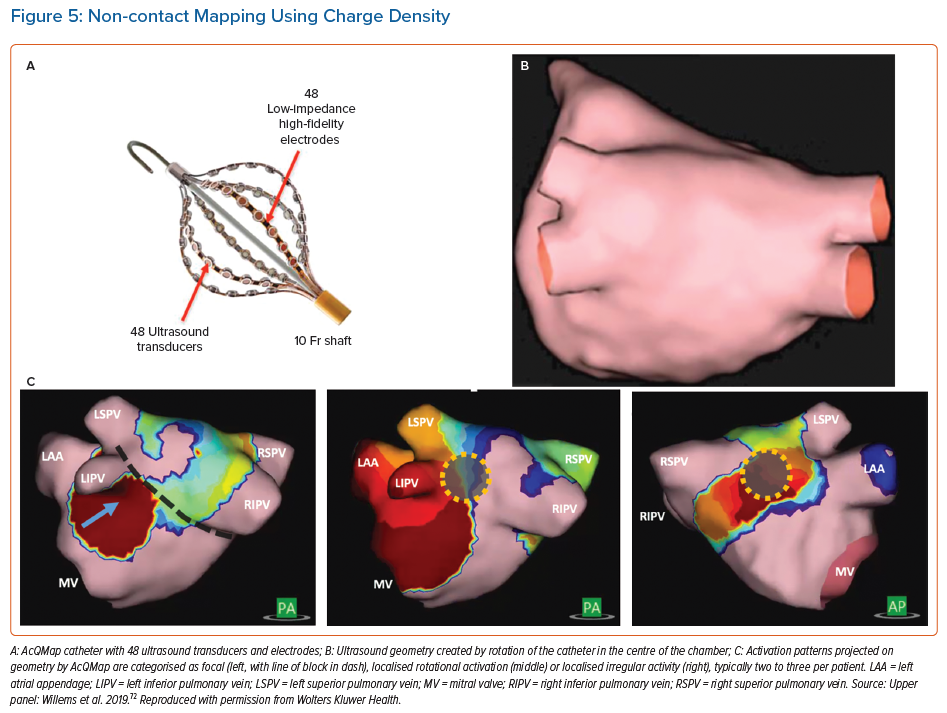
The AcQMap (Acutus Medical) catheter incorporates 48 ultrasound emitters used in real time to generate a 3D anatomy by rotation of the assembly in the centre of the atrial chamber (Figure 5). The physical principle that underpins this approach is that the charge-layer is the true source of the cardiac field, so that calculated charge density provides the most accurate source localisation. Unipolar electrograms (150 ks−1) are acquired and the charge density calculated at fixed times using a governing Poisson formulation and displayed as a movie on a dedicated console (Acutus).
The signals acquired provide acceptable correlations against contact electrograms (correlations >0.80).48,49 The system identifies areas in AF which exhibits localised rotational activity, focal beats and localised irregular activity, the most common patterns. Ablation at these areas has shown 72.3% freedom from AF at 12 months compared to PVI alone (Table 1) in an international multicentre series.50
Non-invasive Body Surface Mapping
Electrocardiographic Imaging
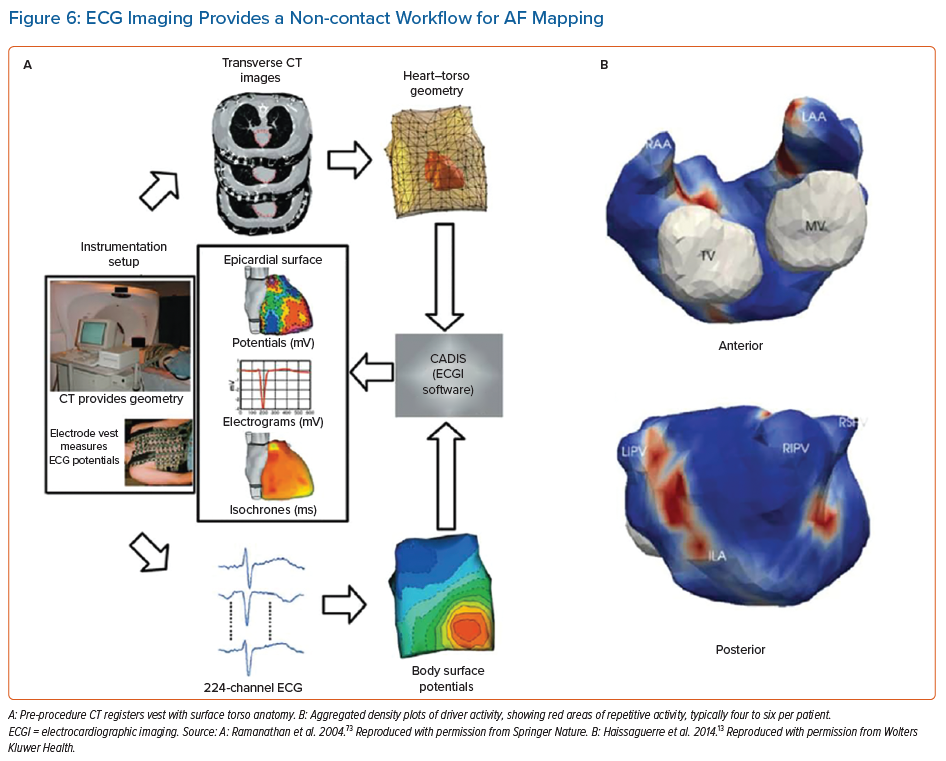
The use of non-invasive body surface mapping using the inverse solution can resolve cardiac electrograms from multipolar ECGs acquired from the chest wall. The most studied approach clinically involves a 252-electrode surface vest (ECVUE, CardoInsight, Medtronic), which is placed on the patient before they undergo a non-contrast thoracic computed tomography scan to obtain high-resolution 3D images of the individual biatrial geometry and the relative electrode positions via segmentation.
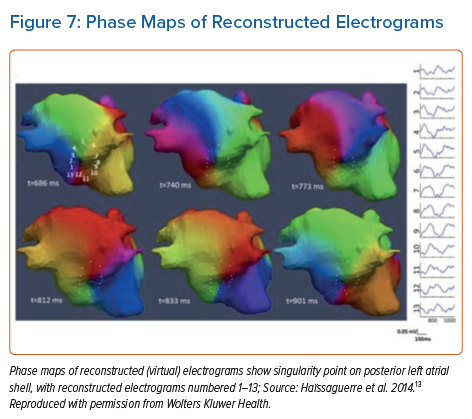
The system reconstructs biatrial unipolar electrograms from torso potentials using mathematical computation. Activation maps are then computed by using the traditional unipolar electrogram intrinsic deflection-based (−dV/dTmax) method. Surrogates of the depolarisation and repolarisation wave fronts have also been computed from the isophase values equal, respectively, to π/2 and –π/2.13 Movies of activation and/or phase can then be used to guide ablation (Figures 6 and 7).
ECGI maps are reported to show ‘driver domains’ bi-atrially, which when targeted may reduce the complexity of AF leading ultimately to atrial tachycardias. This approach has been associated with higher freedom from AF compared to a historical stepwise ablation cohorts, although with up to a 50% recurrence rate with atrial tachycardia.13,51
Body Surface Mapping
A related technique uses body surface recording from a reduced 67 lead recording system without using CT scans to localise electrodes and has demonstrated singularity points on the body surface which become stable when filtered at the highest dominant frequency.52
Studies confirm the presence and detection of AF drivers using this approach when compared to panoramic basket catheters and ablation outcomes using this methodology either as a standalone or in combination with invasive mapping methods are awaited.53,54
Promising Novel Methods
The plethora of technologies seeking to identify AF mechanisms to guide ablation meet the unmet need to extend beyond PVI to treat patients with persistent AF. Another such system with similar early promising data is representation of electrical tracking or origin mapping, which uses a high-density spiral catheter (AFOCUS II catheter, St Jude Medical) to identify spatiotemporal stability in persistent AF.55 Aside from novel mapping systems to visualise AF propagation, using an electrogram visualisation tool already available in CARTO (Ripple mapping), organised activation patterns detected by the Orion basket catheter in Rhythmia (Boston Scientific), consistent activation patterns detected on a PENTARAY and frequency analysis of electrograms have also been reported.56–60
Future Directions
Despite decades of research into fibrillatory propagation, clinical translation into therapy beyond PVI remains debated. Any AF mapping strategy must be explicable and confer additive value. Future AF mapping research must thus explain the interaction with pulmonary-vein-based mechanisms. Although AF termination is increasingly reported as an endpoint of these studies, it remains unclear whether this acute endpoint confers long term clinical benefit. How and why AF terminates in some patients but not others is an area we feel is vital towards developing a common vocabulary and framework to understand AF. Finally, as ablation technologies themselves are rapidly evolving to be more destructive yet safer, it is essential to develop robust endpoints and efficient approaches to map and ablate mechanistically important areas. We believe that better patient selection, by non-invasive imaging and body surface techniques, will help improve the impact of mapping studies in the future to those patients for whom an empirical lesion set, such as PVI is insufficient and better define substrate at an individual patient level.61
Conclusion
There is considerable interest in mapping AF to improve the results of anatomically based ablation in patients with persistent AF. A plethora of basic science as well as clinical studies support the role of spatial regions in sustaining AF. Studies are needed to clarify why ablating putative drivers produce widely differing results between centres, which has limited the ability of randomised trials to show benefit. The field could be advanced by identifying patients most likely to benefit from driver ablation, to standardise mapping and interpretation across centres, and to standardise strategies for ablation of potential drivers. Together, such studies may enable more precise map-based phenotyping of AF patients, and hence improved outcomes from ablation and pharmacological therapies.
Clinical Perspective
- Standard pulmonary vein isolation and empirical ablation strategies approach a ceiling of efficacy in persistent AF.
- AF mapping is increasingly used to identify putative areas of mechanistic importance that often reside beyond the pulmonary veins.
- Many mapping approaches exist, each with their own limitations and strengths.
- Similarities are emerging amongst the patterns observed between techniques and direct comparison to gold standard ex vivo techniques suggests patterns are real.
- Clinical outcome data are promising when compared to pulmonary vein isolation alone but yet to be confirmed in prospective randomised control trials.