There are numerous conglomerations of autonomic ganglia on the epicardial surface of the heart, known as ganglionated plexi (GP). These GP have been shown to play a significant role in different arrhythmias, including AF. As such, GP ablation has become an adjunctive procedure in the treatment of AF. This review will present the current data on the significance of GP in arrhythmogenesis and will discuss the role of GP ablation in the treatment of AF and other diseases.
GP Anatomy and Physiology
The heart is innervated by both the extrinsic (central) and the intrinsic cardiac autonomic nervous system (CANS). The extrinsic CANS consists of the ganglia in the brain or along the spinal cord, where the cell bodies reside, as well as their axons en route to the heart. The intrinsic CANS is comprised of an extensive epicardial neural network of nerve axons, interconnecting neurons and clusters of autonomic ganglia, known as GP, not only on the atria, but also on both ventricles. These GP, most of which are embedded within epicardial fat pads, vary in size, from those that contain just a few neurons, to those that contain over 400 neurons.1,2 Notably, the highest density of autonomic innervation is found at the posterior wall of the left atrium, particularly at the pulmonary vein–atrial junction.2 Several studies have demonstrated that the GP are composed of a heterogeneous population of neurons, including efferent, afferent and interconnecting neurons, the latter group comprising the majority of the neural elements within the GP.3 From a physiological point of view, GP contain both sympathetic and parasympathetic elements, as well as a variety of neuropeptides and neuromodulators, including calcitonin gene-related peptide, vasoactive intestinal polypeptide, substance P and nitric oxide.4,5 The role of each of these neuropeptides has not been fully elucidated. Importantly, the GP serve as the communication centers between the intrinsic and the extrinsic CANS, coordinating the response to afferent and efferent neural trafficking, to control regional electrophysiological, vascular and contractile function.6
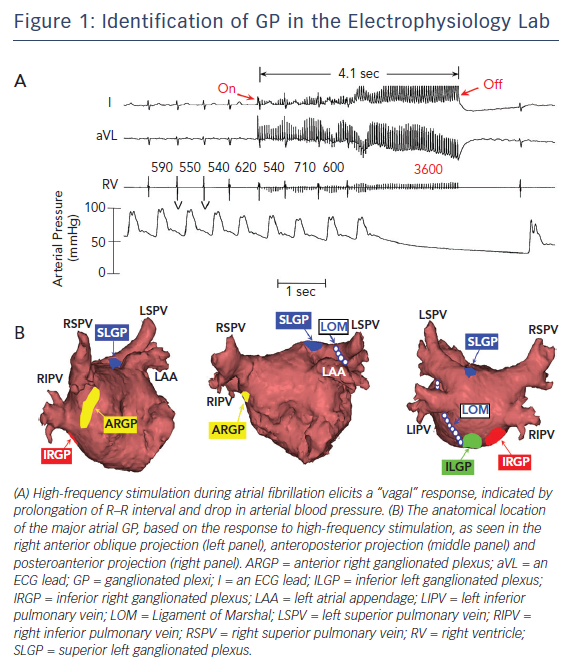
Anatomically, the four major atrial GP are located in close association to the pulmonary veins (PVs) and each innervate one of the four PVs, as well as the surrounding atrial myocardium.1,2 These GP can be identified during electrophysiological study by applying high-frequency stimulation (HFS) (20 Hz) at the respective anatomical locations.7,8 A positive response is defined as an increase in the R–R interval by >50 % during AF8 (Figure 1A). Based on the response to HFS and their location in reference to PVs, the Oklahoma group renamed the major atrial GP for better communication among clinical electrophysiologists. The anterior right GP is located immediately anterior to the right superior PV and often extends inferiorly, to the region anterior to the right inferior PV (Figure 1B). The superior left GP is located at the roof of the left atrium, 1–2 cm medial to the left superior PV (Figure 1B). The right and left inferior GP are located at the inferior aspect of the posterior wall of the left atrium, 2–3 cm below the right and left PVs, respectively (Figure 1B).8 The ligament of Marshal, located between the left atrial appendage and the left superior PV, also contains autonomic neurons. There are multiple interconnections among the GP, converging to the sinus node and atrioventricular (AV) node through the anterior right GP and inferior right GP, respectively.9 Similar neural pathways connecting the GP with the PVs, AV node and sinus node exist in humans.10
GP Ablation for AF
Recent experimental and clinical studies have established the important role that the intrinsic CANS plays in the initiation and maintenance of AF.11 Variations in autonomic tone in humans12 and hyperactivity of the GP in ambulatory dogs13 are commonly observed before episodes of paroxysmal AF. In canine models of AF, GP electrical stimulation that did not excite the atrial myocardium (20 Hz, 0.1 ms pulse width) and produced focal firing from the PVs,14 while injection of acetylcholine into the GP induced, within minutes, focal firing originating from the adjacent PV and sustained AF.15 In addition, it has been shown that PV myocytes have cellular electrophysiological properties distinctive from the adjacent atrium, which facilitate the induction of AF. In these experiments, a shorter action potential duration and greater sensitivity to autonomic stimulation of canine PVs led to triggered firing, similar to the focal firing observed in patients with paroxysmal AF.16,17 Moreover, the autonomic neural activity recorded from the anterior right GP increased hour by hour during 6 h of rapid atrial pacing, suggesting that GP may provide not only the trigger for AF initiation, but also the substrate for AF maintenance.18 In support of this notion, GP ablation reversed acute autonomic remodelling (shortening of atrial effective refractory period and increased inducibility of AF) induced by 6 h of rapid atrial pacing in a canine model of AF.19 Therefore, targeting the GP with ablation in humans appears to be a promising strategy.
Autonomic denervation is common following PV isolation20–22 and has been associated with decreased risk of AF recurrence.20,23,24 Using differential PV isolation and GP ablation, Lemola et al. demonstrated that intact PVs are not needed to maintain experimental vagal AF, whereas ablation of GP prevents AF.25 The same group of investigators showed that PVs play a minor role in AF induced by chronic rapid atrial pacing, whereas intact GP play an important role in AF maintenance in the presence of rapid atrial pacing-induced remodelling.26 It should be noted, however, that the specific neural elements within the GP (efferent, afferent or interconnecting neurons) that are responsible for the beneficial effects of GP ablation remain to be determined. Clinical studies showing that complete electrical isolation of the PVs may not be necessary for maintenance of sinus rhythm support the aforementioned experimental data.27–30 These investigations suggest that the interruption of axons from these hyperactive GP to the adjacent PVs may also contribute to procedural success. It cannot be overemphasised that PV isolation transects at least three of four major atrial GP at the PV–atrial junction (Figure 1B) and numerous autonomic nerves. Thus, the contribution of autonomic denervation to the efficacy of PV isolation cannot be overlooked.
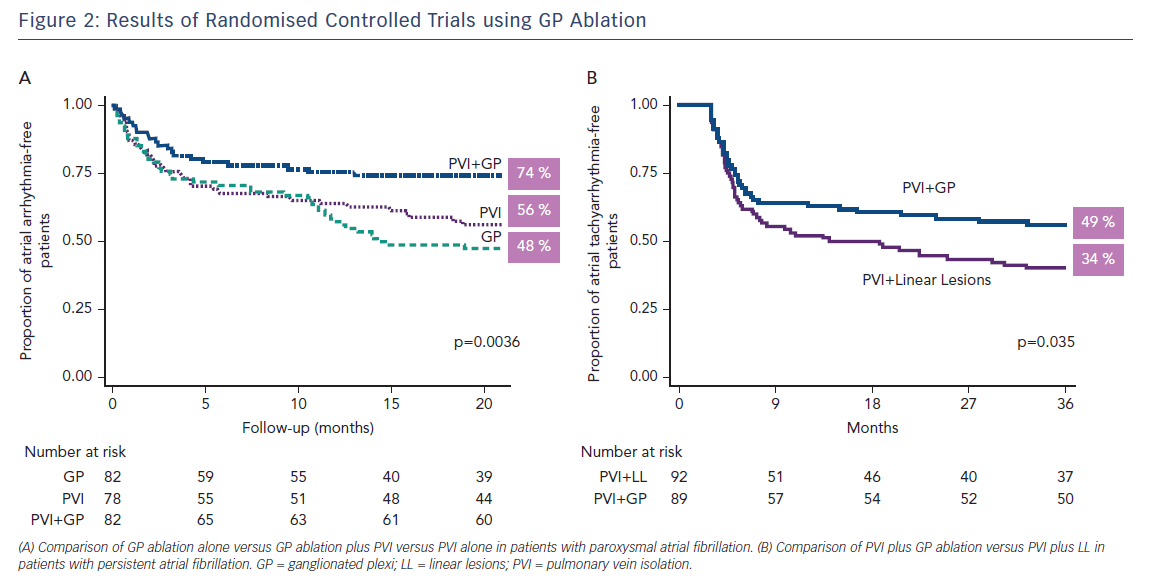
Recent small, randomised trials and cohort studies in which GP ablation was performed either in addition to PV isolation8,31 or as a stand-alone procedure32–34 support the notion that GP play an important role in the pathogenesis of AF. In these studies, the overall success in eliminating AF was increased with the addition of GP ablation by approximately 25 %,8,31 whereas GP ablation alone in patients with either paroxysmal or persistent AF was successful in 71–86 % of the patients.32–34 The largest randomised clinical trial aiming to answer the question of whether the addition of GP ablation to PV isolation improves the success of AF ablation in patients with paroxysmal AF and whether GP ablation alone is as effective as PV isolation was recently published.35 In this study, 242 patients with paroxysmal AF were randomised to conventional PV isolation, PV isolation plus GP ablation and GP ablation alone and were followed for at least 2 years. Freedom from atrial tachyarrhythmias was achieved in a similar number of patients in the PV isolation and GP ablation groups (56 % and 48 %, respectively), and in a significantly greater number of patients in the PV isolation plus GP ablation group (74 %; p=0.004 by log-rank test), lending further support to the important role of intrinsic CANS in the pathophysiology of AF (Figure 2A).
Despite our better understanding of the anatomy and physiology of the major atrial GP, the best GP ablation technique remains to be determined. GP ablation can be performed either empirically, at their presumed anatomical locations,35 or the GP can be identified by applying high-frequency stimulation, as described by the Oklahoma group.8 A major limitation of GP ablation is that despite their consistent location adjacent to the PV–atrial junction, the extent of each “hyperactive” GP that needs to be ablated to treat AF is largely unknown. It should be noted that while the vagal response to HFS is relatively specific, it lacks sensitivity.36 In an attempt to define the best GP ablation technique, Pokushalov et al. randomised 80 paroxysmal AF patients to: (1) selective GP ablation guided by vagal responses induced by HFS (20 Hz); and (2) ablation of GP selected by their presumed anatomical locations.33 AF free rates at a mean of 13 months of follow up were 42.5 % in patients with high-frequency stimulation-guided GP ablation and 77.5 % in patients with anatomic GP ablation (p=0.02). This difference may be explained by the fact that significantly more radiofrequency applications were delivered in the latter group, covering a larger area for each GP. Another possible explanation is that continuous HFS identifies GP sites with inputs to the AV node (a positive HFS response is essentially an AV nodal response), whereas logically it makes more sense to target GP with inputs to the PVs, as PV ectopy is widely accepted as the predominant trigger for paroxysmal AF.11 HFS synchronised to the local atrial refractory period resulting in activation of the autonomic neural elements, as previously described in animals37 and humans,10 may produce better results, although this technique requires further research. Moreover, it should be noted that HFS without concomitant GP ablation has been shown to increase early but not late recurrences in patients with persistent AF.38
While the debate regarding the best substrate modification techniques in addition to PV isolation in patients with persistent AF continues,39,40 GP ablation has also been used in this patient population. In a randomised study including 264 patients with persistent or long-standing persistent AF, GP ablation as an adjunct to PV isolation resulted in higher rates of sinus rhythm maintenance at 3 years (49 %) compared to PV isolation plus left atrial linear lesions (34 %)41 (Figure 2B). In addition, left atrial tachycardias were less common with PV isolation plus GP ablation than with PV isolation plus linear lesions. In another study, GP ablation alone in patients with drug-refractory long-standing persistent AF resulted in less optimal results (38 % sinus rhythm maintenance at 2 years).42 Collectively, these studies provide further support to the autonomic hypothesis for AF, which states that GP hyperactivity is most important in the early stages of AF, while its importance may diminish with progression of the disease to more advance stages and the development of atrial remodelling and fibrosis.36
Surgical techniques have also used the combination of PV isolation with GP ablation.43 Minimally invasive thoracoscopic surgical ablation procedures combining epicardial PV isolation with GP ablation have been shown to achieve freedom from atrial tachyarrhythmias of approximately 80 %, without major adverse cardiac events, at 1 year of follow up.44–46 However, a recent large randomised controlled trial of surgical GP ablation in patients with either paroxysmal or persistent AF and enlarged atria (43 % patients with left atrial volume index >40 mL/m2) or prior failed catheter ablation (25 % patients) showed no benefit of adjunctive GP ablation in this patient population and increased complication rates.47 The lack of benefit of GP ablation in this patient population with advanced AF can be explained by the notion that the role of the intrinsic CANS in the pathogenesis of AF diminishes over time, with atrial remodelling and fibrosis having a more prominent role as the disease progresses.36 Another plausible explanation is that AF is a heterogeneous disease, and the current classification to paroxysmal and persistent AF does not distinguish between the different electrophysiological substrates responsible for AF in each patient. In the future, it may be possible to develop biomarkers, including recordings from single neuron recordings from the GP,48 which would provide a mechanistic approach to AF ablation in each patient, such as those who would mostly benefit from GP ablation. This approach in turn may provide the basis for patient-tailored AF treatment.
GP Ablation for Vasovagal Syncope
Vasovagal syncope is the most common form of neurally mediated syncope.49 Although the pathophysiology of vasovagal syncope is still controversial, the current thought is that it is related to prolonged orthostatic stress, which causes increased peripheral venous pooling with a subsequent fall in venous return to the heart, which in turn results in activation of ventricular mechanoreceptors and a sudden increase in the afferent neural traffic to the brain. The final result is sympathetic withdrawal and parasympathetic enhancement, which manifests as hypotension (vasodepressor type), bradycardia (cardioinhibitory type) and syncope.49 Thus, vasovagal syncope is a disorder caused by an abnormally amplified autonomic reflex involving both sympathetic and parasympathetic components. The treatment of vasovagal syncope includes beta-blockers, alpha-agonists, mineralocorticoids, selective serotonin reuptake inhibitors and dual-chamber pacemaker implantation, but studies of these modalities has provided mixed results.49,50 In contrast to pharmacological therapy and pacemaker implantation, GP ablation for vasovagal syncope provides a means of targeting the root of the problem, which includes disturbances of the intrinsic CANS.51 GP ablation for vasovagal syncope was first introduced by Yao et al., who reported their initial experience on 10 patients with highly symptomatic vasovagal syncope.52 Selective high-frequency stimulation-guided GP ablation resulted in an impressive amelioration of their prodromal symptoms and no recurrence of syncope over 30 months of follow up.52 The therapeutic effects were attributed to autonomic (parasympathetic) denervation, as indicated by an increase in the mean heart rate and a decrease in the high-frequency component of the heart rate variability 3 and 12 months after ablation.52 It should be noted that all 10 patients received “partial autonomic denervation”, as GP were ablated only in the presence of a positive HFS response. This approach resulted in three, six and one patients receiving one, two and three GP ablations, respectively, with the left superior GP being the mostly commontly ablated GP.52
The same group recently reported their long-term results of GP ablation in a larger cohort of patients with vasovagal syncope.53 In this study, 57 consecutive patients with highly symptomatic vasovagal syncope received either anatomical (sequential ablation of all four major atrial GP in their presumed anatomical locations) or high-frequency stimulation-guided GP ablation. During a mean of 36 months of follow up, 91 % of patients remained syncope-free and prodromes were markedly attenuated.53 There were no differences between anatomical and high-frequency stimulation-guided GP ablation in terms of syncope or prodromes, while anatomical GP ablation resulted in a significant decrease in the procedure and fluoroscopy time.53 Similar results were obtained by Pachon et al., who performed extensive right and left atrial ablation guided by spectral analysis for “AF nests” to achieve vagal denervation in patients with vasovagal syncope (40 of 43 patients free of syncope after a mean of 45 months of follow up).54 Although the extent of GP ablation required to treat vasovagal syncope remains to be detemined, collectively, these results indicate that GP ablation is an effective and safe treatment option for patients with refractory vasovagal syncope and call for further randomised controlled trials to confirm the efficacy of this novel treatment.
The Future of GP Ablation
GP ablation appears to be a safe and efficacious adjunctive technique to improve outcomes of PV isolation in patients with paroxysmal AF. In addition, promising results were obtained with GP ablation in patients with vasovagal syncope. Nonetheless, some important questions remain unanswered. First, the long-term (e.g. 5 years) outcomes of GP ablation have not been studied. Second, the mechanism by which GP ablation results in improved outcomes is not completely understood. Although autonomic innervation returns approximately 3–6 months after GP ablation in patients with AF,24 GP ablation improves outcomes up to 24 months after the procedure.35 These results are consistent with a recent autonomic neuromodulation study, in which botulinum toxin injection into the epicardial fat pads in patients undergoing cardiac surgery led to a marked decrease in the incidence of atrial tachyarrhythmias both at 30 days and 1 year of follow up, despite recovery of heart rate variability parameters indicating reinnervation after approximately 6 months.55 Further insight into this unexpected long-term benefit was provided by a recent animal study, in which temporary suppression of major atrial GPs by botulinum toxin injection prevented autonomic remodelling and resulted in long-term suppression of AF up to 3 months.56 Specifically, the parasympathetic neural elements in GP and the sympathetic neural elements in atrial myocardium were reduced by botulinum toxin. These results highlight the critical role of GP in AF progression. Finally, the optimal technique to perform GP ablation as well as the extent of GP ablation required to improve outcomes remain to be determined. Further research needs to be directed toward identifying those patients who are most likely to benefit from GP ablation, and the extent of GP ablation required to improve outcomes. Direct visualisation of the GP using I-123-metaiodobenzylguanidine imaging57 may provide an innovative way to assess the autonomic tone before ablation, the extent of atrial denervation, and correlate with clinical outcomes.
Given that many other arrhythmias are related to autonomic imbalance, including inappropriate sinus tachycardia and outflow tract ventricular premature complexes and/or tachycardia, GP ablation may offer an alternative way to target these diseases with fewer side effects. Further studies are warranted to investigate the utility of GP ablation in these conditions.