AF is a complex clinical entity which remains a difficult condition to durably treat in the majority of patients despite an improved understanding of its pathogenesis in the past two decades. AF is the most common sustained arrhythmia and is estimated to affect 37.5 million adults worldwide, with projections for its prevalence to continue to rise, corresponding with the increasing frequency of the risk factors related to its development.1 AF diagnosis and treatment is associated with substantial financial cost, morbidity and mortality.2–6 For these reasons, finding effective management strategies remains paramount.
Over the decades, the management of AF has attempted to evolve to match the increasing understanding of the triggers for its initiation and perpetuation, with a heightened role of catheter-based ablative strategies.7–9 While traditional focus has been on pulmonary vein isolation (PVI), more recent advanced approaches, including isolation of the posterior wall of the left atrium, ablation of the vein of Marshall (VoM), superior vena cava isolation, left atrial appendage isolation, rotor mapping and ablation, non-PV trigger ablation, scar homogenisation and other strategies, have been explored to limit recurrence in those with persistent forms of AF but with only incremental additional effectiveness compared with PVI alone in clinical studies.10–26 More recently, novel energy sources and mapping algorithms have also been explored for AF catheter ablation.27–33 Even with more sophisticated approaches, superior long-term effective treatment for AF above what is achievable with PVI alone has been a challenge.34–38
However, could a key missing link lie outside the heart itself and be nestled within the nervous system? It is well understood that the autonomic nervous system (ANS) plays a dominant role in several arrhythmias, including AF, via a complex network of neural inputs, outputs and plexi, and is often an untargeted trigger in many management modalities.
Modulating, or in some instances eliminating, key neural connections to the heart have been studied as a means to reduce AF burden including vagal nerve stimulation and ablation of ganglionated plexi (GPs) located within or near the heart, but it is not common practice.39–45 As the techniques for ablation of GPs are relatively straightforward and have more recently become better elucidated with emerging operator experience with cardioneuroablation in selected patients with vasovagal syncope, interest in GP ablation has grown among the electrophysiology community as an adjunctive approach to PVI for catheter ablation of AF patients.46–49 However, there are many questions that need to be examined surrounding neuromodulation endpoints in catheter ablation procedures.50 In this review, we explore the potential role of neuromodulation in the management of AF, including its principles, key technical considerations, shortcomings and potential future advances.
The Heart’s Little Brain: Regulator of Heart Function and Trigger of Arrhythmias
Through centuries of observation and experimentation, the intricate hierarchy of reflex arcs connecting higher centres in the medulla, hypothalamus, thalamus, amygdala cerebral cortex and thoracic ganglia with the heart have been elucidated. The extrinsic domain of the cardiac nervous system includes the autonomic ganglia and nerve fibres en route to the heart. A ganglion is a cluster of neuronal cell bodies outside the brain.51–53 In the ANS, efferent axons from the ganglion to the effector organ are called postganglionic nerve fibres. While postganglionic nerve fibres of the sympathetic division extend from the sympathetic chain or paravertebral ganglia to the heart, ganglia of the parasympathetic division and their associated clusters of nerve cell bodies (GPs) – which are considered intrinsic – are distributed mainly within the epicardial area. From there the post-ganglionated intrinsic nerves extend towards specific atrial or ventricular regions around the sinoatrial node, the roots of caval and PVs, and near the atrioventricular node.
Location of Ganglionated Plexi Within the Heart
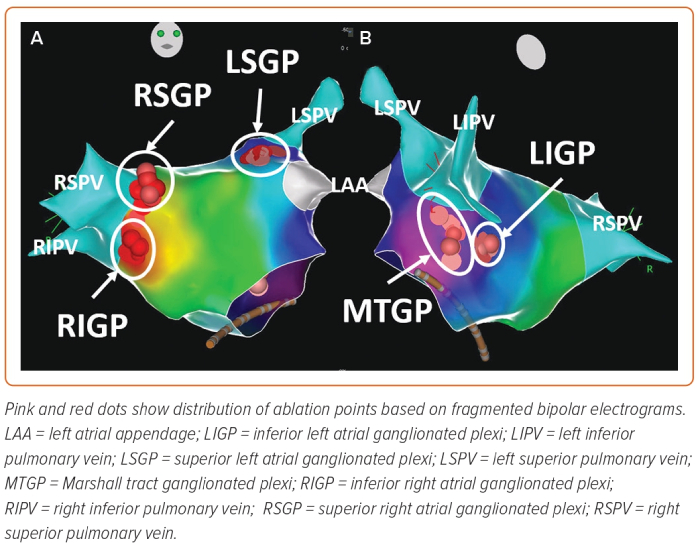
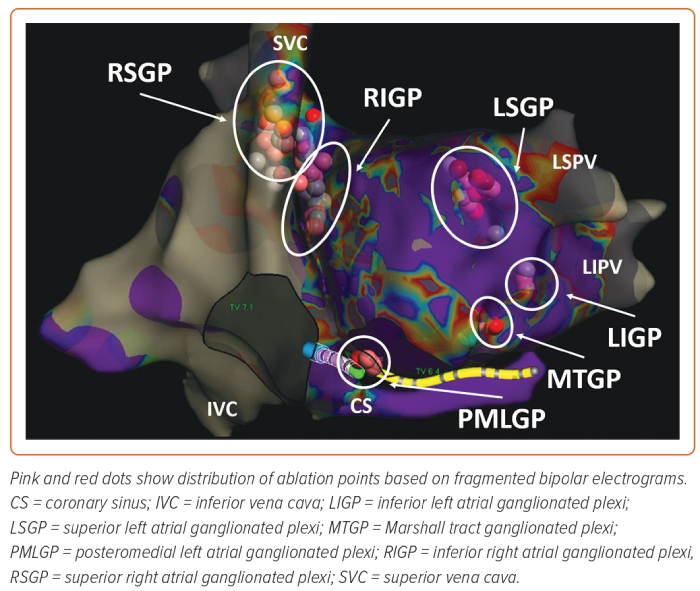
Several nests of ganglionated plexi have been identified within the heart via gross anatomical and histological studies which have defined the distribution of intrinsic cardiac ganglia in experimental and clinical studies:
- the superior (anterior) right atrial GP (RSGP) located on the posterosuperior surface of the right atrium (RA) adjacent to the junction of the superior vena cava (SVC) and the RA;
- the inferior (posterior) right atrial GP (RIGP) located adjacent to the interatrial groove;
- the superior left atrial GP (LSGP) on the posterosuperior surface of the left atrium (LA) between the PVs;
- the posterolateral (inferior) left atrial GP (LIGP) is identified on the posterolateral surface of the LA;
- the posteromedial left atrial GP (PMLGP) on the posteromedial surface of the LA; and
- the interatrial septal GP consisting of fusion and extensions of RIGP and PMLGP (Figures 1 and 2).54,55
However, it should be noted that a dense meshwork of neurons has been characterised by immunohistochemical methods as predominantly cholinergic, although adrenergic-, nitrergic- and peptidergic-positive fibres have also been identified within the GPs so it is too simplistic to assume that the GP behave as one unit. Furthermore, the specific neural elements responsible for the ablation outcomes remain unknown.56
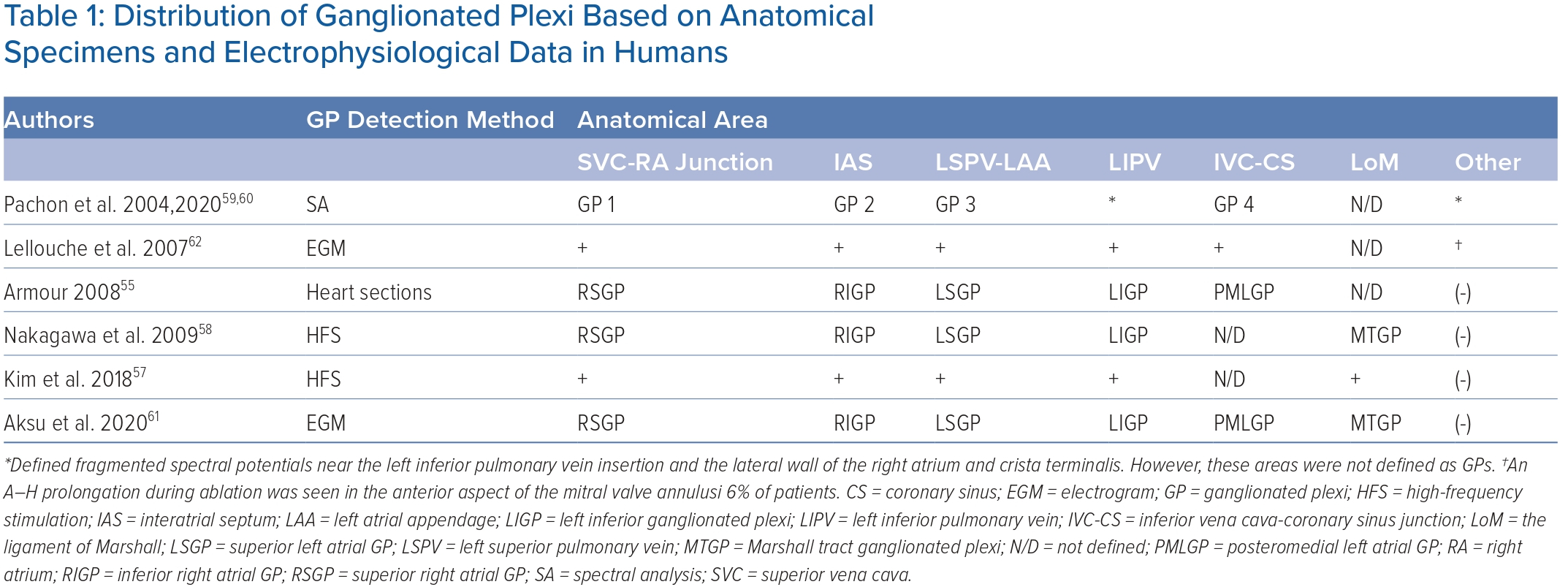
The ligament of Marshall (LoM) is also considered part of the intrinsic cardiac ANS. Cholinergic nerve fibres originating in the LoM were found to innervate surrounding left atrial structures, including the PVs, left atrial appendage and coronary sinus.51 The VoM is one of the structures contained within the LoM and permits access to it through the coronary sinus. However, the precise location and distribution and density of the GP may vary significantly among individuals, making it challenging to predict their precise location based on anatomy alone.57 Table 1 shows the distribution of epicardial ganglia based on anatomical specimens and electrophysiological data.55,57–62
Autonomic Tone, Ganglionated Plexi and Atrial Fibrillation
The role of the ANS as a trigger for the initiation and maintenance of AF is well established. First speculation of this association was highlighted by Coumel et al., who reported a small case series of 18 patients without structural heart disease who had recurrent paroxysms of AF/atrial flutter which appeared to be initiated by sinus rate slowing and atrial coupling attributed to vagal overactivity.52 Derangements in sympathetic tone are also thought to play a central role in AF, possibly via cellular, structural and electrical changes which occur in the setting of states of heightened adrenergic tone, including hypertension, obstructive sleep apnoea and heart failure.53,63
Additional clues to the role of the ANS in the initiation and termination of AF have been demonstrated by circadian periodicity in which episodes of AF are more frequent in the early morning and evening.64,65 Obstructive sleep apnoea (OSA) shares similar risk factors and may be a modifier for AF. Screening for OSA is currently recommended in patients undergoing attempts at rhythm control.66 In a study of 101 patients, Mohammadieh et al. found that paroxysmal atrial fibrillation (PAF) patients with OSA had increased parasympathetic tone and relative reduction in sympathetic modulation during non-REM sleep when assessed by frequency-domain analysis of heart rate variability (HRV) suggesting vagal predominance as a contributor to AF in the subpopulation.67 Another key clue of this role is the fact that therapies which blunt components of the autonomic nervous system suppresses AF in both animal and human studies.68–74
Endocardial Mapping of Ganglionated Plexi
From a clinical standpoint, the identification of the GPs and understanding of their anatomic clustering in the electrophysiology laboratory is an important step for targeting potential modulation. A study conducted by Po et al. demonstrated that application of high-frequency stimulation (HFS) of 20 Hz between 10–140 V at a 1–10 ms pulse width in anatomical regions where GP are known to be located resulted in a marked parasympathetic response, which was defined as an increase in the mean R-R interval by 50% during AF.75 A similar method of GP identification using HFS, between 20–50 Hz, between 5–15 V and pulse width of 10 ms, was also shown to be able to precisely locate GP sites.76 In a recently published randomised controlled trial (RCT), Kim et al. compared GP ablation without PVI against PVI in patients with paroxysmal AF.77 To map GPs, two types of HFS techniques were used: synchronised HFS with 10 V, 80 ms duration, 40 Hz to detect the ectopy-triggering GPs and continuous HFS with 10 V, 20 Hz up to 10 seconds to detect the atrioventricular dissociating-GPs. In the GP ablation arm, only ectopy-triggering-GPs were targeted and ablated. The freedom from ≥30 seconds of atrial arrhythmia at 12-month follow-up was 50% (26 of 52) with GP ablation versus 64% (32 of 50) with PVI (p=0.09).
By using spectral analysis, Pachon et al. demonstrated that fibrillar potentials show fragmented and heterogeneous conduction properties and might result from incursions of neural and vascular structures and be used to detect autonomic innervation sites.60 By using a classical band-pass filter setting of 30–500 Hz, Lellouche et al. analysed electrogram characteristics based on parasympathetic response during radiofrequency (RF) application and demonstrated that the best single predictor of parasympathetic response during RF application was the presence of at least four electrogram deflections at the ablation site.62
Stirrup et al. used 123I-metaiodobenzylguanidine (123I-mIBG) solid-state single-photon emission CT to map left atrial GPs, non-invasively.78 All patients underwent cardiac CT as part of standard clinical care for delineation of LA and pulmonary venous anatomy prior to PVI. Following registration, the CT-derived LA segment was used as an anatomical constraint to define a region of search around the LA endocardium to facilitate identification of focal mIBG uptake adjacent to the atria. Focal increased mIBG activity within the search region was automatically overlaid on the CT-derived left atrial surface, generating a hybrid 3D image of left atrial innervation and anatomy. I-mIBG LA uptake areas were recorded and correlated with HFS. A total of 73 I-mIBG LA uptake areas were identified, of which 59 (81%) were HFS positive. The likelihood of this increased with reader confidence (92%).
More recently, our group demonstrated that RF catheter ablation guided by the identification of complex fractionated left atrial electrograms during sinus rhythm using 3D electroanatomical mapping correlated highly with the distribution of successful GP ablation sites without need for HFS, resulting in significant simplification of procedural workflow to add adjunctive GP ablation to PVI.62,79–82 Briefly, after 3D mapping both atria, fragmented bipolar endocardial atrial electrograms are evaluated for the number of deflections at filter settings of 200–500 Hz by using the ablation catheter. The electrograms demonstrating greater or equal to 3–4 deflections in regions which are anatomically consistent with GP sites are tagged as ablation targets. Despite all these specialised techniques, it should be noted that the largest RCT to so far examine the role of adjunctive GP ablation to standard PVI used an anatomical approach for GP ablation.83
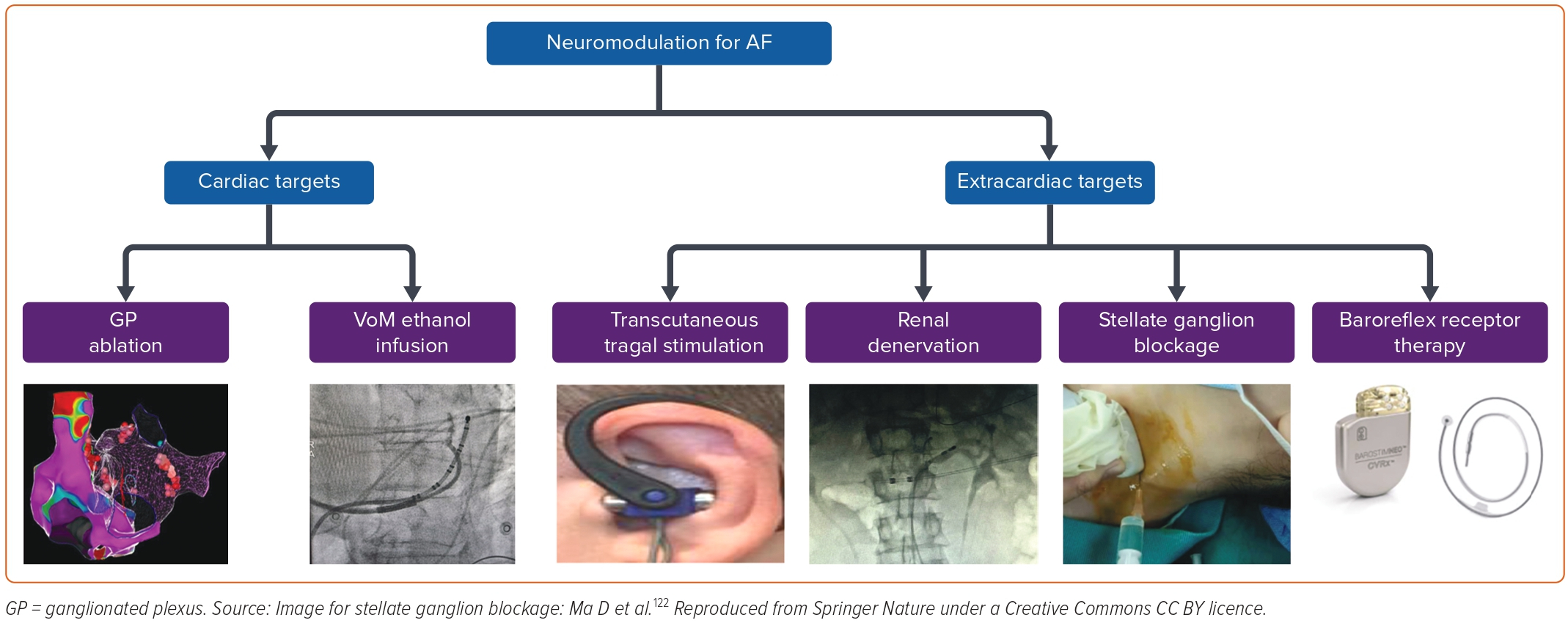
Neuromodulatory Strategies in the Management of Atrial Fibrillation
Several non-catheter neuromodulatory techniques have been explored in the management of AF both as alternative or adjunctive therapies to PVI with varying degrees of success. These include procedures targeting neural inputs within the heart and those in extracardiac structures (Figure 3). Targeting autonomic ganglionic plexi and alcohol injection in the VoM have yielded moderate results within cardiac structures. Techniques focused on neuromodulation extrinsic to the heart include transcutaneous vagal nerve stimulation, renal nerve denervation, stellate ganglion block and baroreflex receptor therapy.
Ganglionated Plexus Ablation
Several clinical trials have explored the use of GP ablation in the management of AF. While the results of these studies have been generally quite favourable, with decreased rates of AF recurrence when compared to PVI alone, it is worth noting that there is not a standardised method for performing these ablations.75,83–86 Additionally, as a stand-alone treatment strategy for AF, GP ablation success rates have been lacklustre. Specifically, in one study examining the long-term impact of GP ablation during a 3-year follow-up period showed that isolated GP ablation was associated with significantly lower rates of freedom form atrial tachyarrhythmias (AT) without antiarrhythmic drug therapy when compared to circumferential PVI (34.3% versus 65.7%, p=0.008).87 Several pooled analyses, including a recent RCT-only meta-analysis, have found that GP ablation as an additive strategy to standard PVI may be more beneficial in patients with paroxysmal rather than persistent AF.88
One of the factors that may limit the durability of GP ablation on freedom from AF may potentially be the phenomenon of nerve regeneration and reinnervation post-ablation. In one small canine study in which epicardial GP fat ablations were performed, features of restoration of vagal effects were noted within 4 weeks post-procedure and were suggestive of reinnervation.89 Long-term recovery of autonomic tone has also been observed following cardioneuroablation for cardioinhibitory vasovagal syncope and may affect the durability of those therapeutic interventions as well.90–92 However, given the additive benefit of combined GP ablation and PVI has been demonstrated to be long-term in comparison to PVI alone, the nerve regeneration hypothesis may not be universally true, or it may suggest that additional factors independent of nervous inputs may be involved in this population and warrants further investigation.
According to the published studies, an RF current should be applied in a point-by-point fashion in power-controlled mode with an open irrigated-tip catheter. RF energy should be limited to 30–35 W along the left atrial posterior wall and roof and to 40 W in the remaining areas for a duration of at least 30 seconds at each site.
Pulsed electric field (PEF) ablation is currently being investigated for human use for PVI and linear atrial ablation. Because every tissue has a different specific field threshold that induces necrosis, PEF-based irreversible electroporation induces selective myocyte necrosis without collateral damage to other tissues such as the oesophagus, the phrenic nerve, or the endothelial cells. However, in clinical cases and animal studies, transient bradycardia and even atrioventricular block have been observed with endocardial pulsed field applications in the LA. Wei et al. showed that nerves treated with irreversible electroporation were damaged after immediate direct injury with a full recovery after 2 weeks.93 However, in an open-chest canine model, saline irrigated PEF with a changed setting (1,000 V, 100 ms) were directly delivered to visualised epicardial GP regions (LSGP, RSGP, LOM GP, oblique sinus GP and transverse sinus GP) using an anatomy-guided approach. Histological examination showed preserved function and structure of the atrial myocardium but also absence in acute structural change to nerves and GP. However, the local atrial effective refractory period (AERP) increased by 12–29% at different atrial sites following PEF applications suggesting perturbation of cardiac parasympathetic innervation in the acute model.94 In a clinical comparison of 31 patients who underwent PVI using a lattice-tip catheter and PEF energy versus 13 patients who underwent PVI using RF energy, alteration in sinus node and AV node function was observed more frequently in the RF ablation group. Further, earlier recovery of GP function was noticed only in the PEF group, suggesting that RF ablation may be more advantageous in terms of long-term durable effectiveness for GP ablation.95
Vein of Marshall Ethanol Ablation
The VoM is one of the structures contained within the LoM and is embryologically derived from the left superior vena cava. Autonomic system inputs (parasympathetic and sympathetic) are part of the complexity of the LoM and play a role in the initiation and maintenance of AF.51,96 Ablating these nervous inputs and abolishing vagal responses using ethanol infusions in the VoM has been shown to be successful in animal and human studies.97
The recently published VENUS trial showed that for individuals who had successful infusion of ethanol to the VoM at the time of AF ablation (PVI and non-PV sites), AF/AT recurrence rates 6–12 months post-procedure were significantly reduced with an (OR 0.57, 95% CI [0.37–0.90]), when compared to those who had PVI catheter ablation alone.13,98 One of the proposed explanations for this technique reducing AF recurrence rates is that infusion of ethanol in the VoM leads to disruption of parasympathetic nerve connections that are key to triggering AF.99 However, it has been noted that there is significant heterogeneity on the impact of VoM infusion on rhythm control depending on the lesion sets performed at the time of AF ablation and the procedural endpoint used.
A subsequent secondary analysis of the findings of the VENUS trial sought to explore this heterogenicity further.100 The analysis showed that freedom from AF/AT was greatest if perimitral block was achieved (54.3% post VoM catheter ablation, 37% post catheter ablation alone, p=0.01) compared to when perimitral block was not achieved (34.0% post VoM catheter ablation, 37.0% post catheter ablation alone, p=0.583). Based on this finding, the authors concluded that using perimitral block as a therapeutic endpoint should be sought at the time of ablation.
Other than ablating the autonomic and the muscular fibres of the LoM, which can be arrhythmogenic per se, VoM ethanol infusion may affect the area around the left inferior pulmonary vein creating a low voltage zone.101 This may facilitate acute and chronic block of the posterior mitral isthmus reducing the post-PVI perimitral flutters. As seen with GP ablation, the role of VoM ethanol infusion appears to be additive, reflecting the multifaceted nature of the pathophysiology of AF.
Transcutaneous Tragal Stimulation
Stimulation of the auricular branch of the vagal nerve via low-level transcutaneous tragal stimulation (LLTS), which is applied to the tragus of the ear, has been shown to be effective at suppressing AF by decreasing sympathetic tone and blunting inflammatory cytokines which may contribute to AF.102–104
In this non-invasive method of neuromodulation, flat metal clips are attached to the tragus and 20 Hz electrical stimuli are applied. In a recently sham-controlled double-blind trial, participants either attached ear clips to the tragus (intervention group) versus the earlobe (sham group) and performed LLTS for 1 hour daily over a 6-month period.39 Participants in the active treatment group had an AF burden 85% lower than the sham group (p=0.01). Although this is a potentially exciting treatment prospect, responses to LLTS may be inconsistent within individual patients and predicting which patients will benefit most from this approach may be difficult.
One proposed novel approach to assist in patient selection and therapy adjustment is examining the burden of P-wave alternans (thought to be generated by the same mechanism as AF) at the time of initial LLTS. Using this method, people who had an increase in P-wave alternans after acute treatment had a lower AF burden at 6 months of chronic LLTS compared to those who did not have an acute increase.40 Further studies are needed to define the role that this strategy will play as a stand-alone AF therapy or adjunct to other treatments including catheter-based therapies. Other considerations such as loss of efficacy due to physiological tolerance, as has been seen with chronic electrical peripheral nerve stimulation, will also need to be examined further.105
Renal Sympathetic Denervation
Another neuromodulation strategy in the management of AF to be recently examined is renal sympathetic denervation (RSD). The renal sympathetic nerves, which are located in the walls of the renal artery, interact closely with the central ANS (which in turn has inputs to the heart) and have been implicated in the development of resistant hypertension and AF. Based on this, it has been hypothesised that ablating these nervous connections could be related to the attenuation of afferent sympathetic input from the aorticorenal ganglion to the central nervous system, as well as attenuation of efferent signalling from the aorticorenal ganglion to the renal parenchyma, reducing renin–angiotensin–aldosterone system activation which alters sympathetic tone and leads to reductions in blood pressure and AF burden.106
The ERADICATE-AF trial was conducted to study this hypothesis further.107 This RCT included 302 patients with uncontrolled hypertension and paroxysmal AF who underwent renal denervation in addition to PVI versus PVI alone. After a 12-month period of follow-up, freedom from AF and AT was noted to be significantly lower in the group undergoing both PVI and renal denervation when compared to the group undergoing PVI alone (72.1% versus 56.5%, p=0.006). This was accompanied by a significant reduction in systolic and diastolic blood pressures in the group undergoing both PVI and renal denervation.
It is worth noting that while these results in blood pressure reduction are much more impressive than those seen at the 6-month mark of the landmark SYMPLICITY HTN 3 trial which compared catheter-based renal denervation to a sham control (which showed no significant change to blood pressure), they align more with those of the recently published long-term follow-up.108,109
Important factors should be taken into consideration when examining the results of the ERADICATE-AF trial, however. First, there was no sham control arm – a limitation noted by the study authors – as well as some uncertainty about the specific mechanism of AF reduction. Renal sympathetic denervation decreased AF recurrence in ERADICATE-AF trial. However, the specific mechanism of AF reduction is not clear. One possibility is that denervation decreases hypertension, which decreases AF burden, or sympathetic denervation directly affects AF burden.
A systematic review and meta-analysis demonstrated a significant reduction of AF recurrence in the PVI + RSD group versus PVI alone (n=223 versus 228; pooled OR 0.63, 95% CI [0.50–0.80]; p<0.001, I2=0.0%) in select hypertensive patients.110 As more data from clinical trials becomes available, RSD may be more favourably considered as a strategy to improve AF burden in selected patients with refractory hypertension for whom AF ablations are planned.
Stellate Ganglion Block
Blocking the stellate ganglion (SGB), a source of major sympathetic input to the heart has been shown to be beneficial in the management of drug- refractory ventricular arrhythmias.111–113 However, the role of this technique has been less well studied in the management of AF.
In a small prospective study, 36 consecutive patients with paroxysmal AF were randomised to transcutaneous SGB using lidocaine or placebo prior to planned PVI. The ability to induce AF, AF duration as well as the atrial effective refractory period (AERP) were evaluated. There was a significant reduction in AF inducibility pre- and post-SGB (100% versus 54%, p<0.01), and a shortening of AF duration 5.5 (3.0–12.0 minutes) – 1.5 (0.0–5.8) minutes (p<0.01) before and after SGB.114
A subsequent RCT involving 200 patients showed significant reductions in AF episodes in the first 24 hours post-surgery in patients undergoing lobectomy who had right-sided SGB when compared to those who did not (3% versus 10%, p=0.045). To validate these findings further, other RCTs are further exploring the role of SGB in the prevention of postoperative AF, such as NCT05357690.
Given the infancy of this technique for AF management, many questions remain unanswered particularly surrounding the duration of its efficacy which may be only a few weeks when used for management of ventricular tachycardia.115 Based on the limited data available and transient nature of its effect, restriction to periods of heightened AF risk such as that in the perioperative period for cardiothoracic surgeries appears to be valid.
Baroreflex Receptor Therapy
Baroreceptors in the carotid sinus and its accompanying reflex arcs play a dominant role in blood pressure homoeostasis through alterations in cardiac contractility, heart rate response and peripheral vascular resistance.116 Abnormalities in the function of these baroreceptors have been implicated in patients with AF and heart failure.117
Animal models have demonstrated that low-level carotid baroreflex stimulation (LLCBS) was successful at suppressing AF and could reverse right atrial remodelling that occurred in the setting of a high right atrial pacing burden.118,119 To date, there are limited human studies examining baroreflex receptor therapy (BRT) for the management of AF, however, its use in patients with heart failure has been explored and was found to be safe and effective in this population via the suppression of central sympathetic outflow.120,121 The role of BRT in the management of AF has yet to be defined, with more data from human studies needed.
Conclusion
The autonomic nervous system is intimately involved in the pathophysiology of AF. Several methods to decrease AF burden via neuromodulation are being explored, some of which have demonstrated potential for clinical effectiveness as adjuncts, namely GP ablation, RSD and VoM ethanol infusion, to established catheter ablation PVI. The prospect of non-invasive techniques, such as transcutaneous tragal stimulation is exciting, but further research in this area is needed to guide clinical application.
Neuromodulation appears to offer at least incremental benefits to existing established management strategies for AF. Importantly, these methods remind us of the complex and multifaceted pathological processes that promote atrial AF and the need for a similar multifaceted approach to its management. 
Clinical Perspective
- The autonomic nervous system plays an important role in AF via a complex network of neural inputs, outputs and plexi.
- Modulating, or in some instances eliminating, key neural connections to the heart may decrease AF burden.
- Targeting autonomic ganglionated plexi and alcohol injection in the vein of Marshall in addition to pulmonary vein isolation may increase AF-free survival.