ICD implantation rates have been increasing over the years due to advancements in technology, expanding indications and a better understanding of the benefits of ICD therapy.1–4
While ICDs are highly effective in preventing arrhythmic death, the psychological impact on patients is a significant consideration.5–7 Patients with ICDs often live with the fear of receiving a shock, which can be a traumatic experience.8 The anticipation of a shock can lead to heightened anxiety and stress. The presence of an ICD can affect a patient’s quality of life.9 Concerns about shocks, and the awareness of having a potentially life-saving device implanted, can impact daily activities and overall wellbeing. Some patients may develop avoidance behaviours, such as isolating themselves socially or avoiding physical activities for fear that exertion might trigger an unnecessary shock. These behaviours can contribute to a diminished quality of life. Living with the knowledge that one is at risk for life-threatening arrhythmias and the potential need for shocks can lead to mood disturbances, including depression and anxiety.10
Several studies discuss strategies for reducing the frequency of ICD shocks, particularly inappropriate ICD shocks. Effective approaches include optimising device programming, managing arrhythmias with medications and performing catheter ablation when necessary.11,12 Recognising the psychological impact of living with an ICD, healthcare providers often emphasise the importance of psychological support.13 This may include counselling, support groups and education about the device to help patients cope with the emotional aspects of their condition.
Studies and clinical practice have shown that patients who have ICDs may experience a psychological phenomenon known as phantom shock (PS).14 It was first described in 1992.15 This phenomenon is when a person perceives having received an ICD shock without any actual shock being delivered. This subject, which intersects cardiology and psychology, is poorly studied. It has been linked to various psychological conditions, but its prevalence, risk factors and frequency of occurrence remain unclear, and require further research.
The pathophysiological understanding of PSs is poor. ICD patients may experience PSs due to anxiety or heightened awareness. It is believed to originate from psychological factors, specifically memory reactivation of traumatic events.16 Our study aimed to gather all available data on this phenomenon. By shedding light on PSs, and incorporating this awareness into medical practice, research and education, the healthcare community can work towards improving the overall wellbeing and quality of life for patients with ICDs. PSs can sometimes lead to concerns about device function or fear of future shocks, potentially affecting patient compliance with medical advice and follow-up appointments.
Methods
We conducted a comprehensive review of medical research from databases, such as PubMed, Embase and Cochrane, up to March 2024. Our focus was on investigating studies related to patients with ICDs and their encounters with PSs. Our data collection encompassed details about the study participants, the frequency of PSs, potential triggers and predictive indicators.
To ensure the credibility of our analysis, we implemented rigorous measures. These included eliminating redundant information, scrutinising titles and abstracts to identify pertinent studies, and carefully examining the full texts of selected research papers. Additionally, we employed specific criteria to assess the quality of each study.
Upon completing these meticulous steps, we carefully examined the data to identify correlations between potential triggers, predictive indicators and the occurrence of PSs in ICD patients. This comprehensive study enabled us to draw meaningful conclusions and provide valuable insights into the understanding of PSs within this specific medical context.
Results
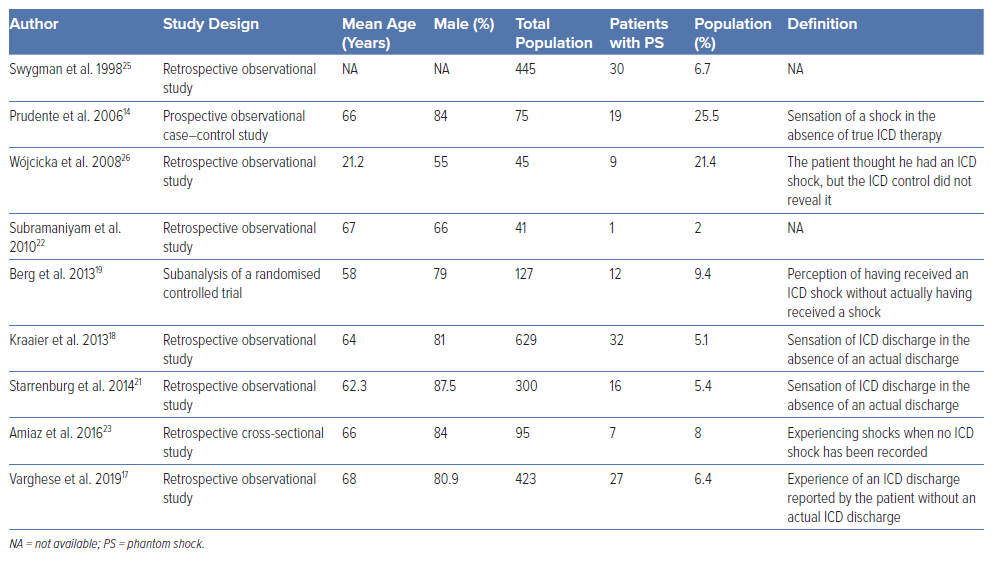
Our search yielded a total of 184 articles from Embase, 140 from PubMed and 15 from Cochrane. Following the elimination of duplicates and a meticulous manual verification process, we pinpointed 21 articles that were relevant to our study. This selection encompassed eight case reports, three reviews and nine pertinent studies.
Definition of Phantom Shock
The definition of phantom shock was consistently similar across all studies: it refers to the sensation of receiving an electrical shock from an implantable device, such as a defibrillator, even though no actual therapeutic shock was delivered by the device.
Incidence and Prevalence of Phantom Shocks Among ICD Patients
The incidence rates of PSs in patients with ICDs have been found to range from 2% to 25.5%. Out of a total of 2,483 participants across all studies, predominantly men, 167 reported experiencing a PS. This translates to approximately 6.7% of ICD patients encountering a PS. However, our analysis was hindered by methodological variations among the nine studies, making it difficult to standardise and interpret the results consistently.
Relationship of Phantom Shocks to Defibrillation Threshold Testing and Actual Shocks
Six studies analysed the occurrence of PSs in patients who had previously received an actual ICD shock. Four of these studies found no significant difference in the occurrence of PSs between individuals who had previously experienced an actual shock and those who had not, except for one that found an association only in primary prevention ICD patients, but not secondary prevention. In contrast, two studies found a relationship to the occurrence of PS in the case of actual shocks in the past, and one of the studies found that there were no PSs in patients who did not experience an actual shock or defibrillation threshold (DFT) testing. It is worth noting that this relationship to DFT testing was investigated in older studies, as in recent years, the practice of DFT testing after ICD implantation has been abandoned, except for specific cases.
Varghese et al. found a significant association between a positive history of appropriate ICD shocks and the occurrence of PS (OR 59.0; 95% CI [17–205]; p<0.0001).17 Kraaier et al. found that prior actual shock had no significance for the occurrence of PS (p=0.23), except in the primary prevention group (p=0.19), and particularly appropriate shock therapy in the primary prevention group (25.0%) versus (13.1%) in the no PS group.18 Therefore, both found a significant association to appropriate ICD shocks. Another significant association was found by Jacob et al. that patients with a history of ICD shock storms were threefold more likely to have PSs (39.5% versus 11.8%; p=0.001).16 No PSs occurred in patients who did not undergo DFT testing or had no history of ICD shock storms. More than half of the patients (61.1%) experienced PSs within 6 months following DFT testing or a recent shock. A history of DFT testing increased the likelihood of a PS to 52%. Even without past shock storms, patients with prior documented DFT testing had 8.61-fold higher odds (p=0.002; 95% CI [2.28–32.58]) of experiencing a future PS. Furthermore, 20 out of 38 patients in the PS group had a prior history of actual shock, and 15 of these experienced ICD shock storms. These patients were 17.71-fold (p=0.000; 95% CI [4.08–76.87]) more likely to experience PSs. All patients with PSs had a history of DFT testing or shock storms.
In a study by Prudente et al., they found that out of 19 patients who reported PSs, eight had never actually received shocks before, while 11 had experienced shocks previously, ranging from one to >10.14 Surprisingly, the number of actual shocks received did not significantly differ between those who reported PSs without prior shocks and those who had received shocks before (χ2=3.8; d.f.=2, p=0.15). In another study by Berg et al., only two out of 12 patients who experienced PSs had received an actual shock before.19 However, the analysis did not show a significant difference between those who had received an actual shock and those who had not (p=0.132). Similarly, Bilanovic et al. found no significant difference in the number of actual shocks between the PS group and the group that actually received shocks (F[1, 15]=0.912; p=0.355).20 However, more participants in the PS group had received an ICD implantation for primary prevention compared with the group that actually received shocks (66.7% versus 12.5%; χ2[1, 17]=5.13; p=0.024). Notably, almost all participants in the PS group had previously received actual shock therapy, except for one.
Relationship of Phantom Shocks to Anxiety
Five studies addressed the relationship between PSs and anxiety. The Hospital Anxiety and Depression Scale (HADS) was the primary tool used to assess anxiety levels in these studies, with the exception of one that used the State-Trait Anxiety Inventory and another that relied on clinically documented anxiety. As is the case for depression, anxiety scores were measured after the occurrence of shock, with the exception of the study that focused on patients with pre-existing anxiety. Two studies showed no association between PSs and anxiety, whereas three studies found that patients with PSs had higher levels of anxiety.
Prudente et al. found that people who experienced PSs showed significantly higher levels of both state (p=0.04) and trait anxiety (p=0.03) compared with those who received actual shocks or no shocks, using the State-Trait Anxiety Inventory.14 Varghese et al., using the HADS, found a significant difference between PSs and no PSs groups in ICD patients.17 The PSs group had 0.0% known anxiety, compared with 2.3% in the no PSs group. The follow-up HADS scores were higher in the PSs group than those without a PS (p=0.04). Patients with a history of PSs showed significantly higher overall anxiety (p<0.0001) using the HADS. Furthermore, Jacob et al. studied pre-existing anxiety and found that patients with PSs had a higher prevalence (23.7%) compared with 3.9 % in patients without PS (p=0.001).16
Starrenburg et al. found no association between anxiety and PSs using HADS and lifetime presence of anxiety by Mini-International Neuropsychiatric Interview (MINI) structural interview.21 Bilanovic et al. found that both the group with PSs and the group without PSs experienced elevated levels of anxiety based on the HADS assessment. The p-value was 0.915, with an F-value of 0.012 and an ηp2 of 0.001.20 However, it is important to interpret these findings with caution due to the underpowered nature of the study.
Relationship of Phantom Shocks to Depression
Seven studies addressed the potential correlation between PSs and depression in ICD patients. These studies used various tools to measure depression, including the Center for Epidemiologic Studies Depression Scale, Zung Self-Rating Depression Scale, MINI, Hamilton Anxiety Rating Scale and HADS, making it difficult to compare results. It is also worth noting that depression levels were measured after the occurrence of shock, except in one study that studied pre-existing depression. The dilemma remains in most studies, as it is uncertain whether PSs caused the depression or if it was pre-existing. While one study found no association between PSs and depression, six studies found a significant correlation between PS and higher levels of depression.
Prudente et al. used the Center for Epidemiologic Studies Depression Scale to assess depression among ICD patients, finding more clinically depressed patients in the PS group compared with the no PS group (p=0.01).14 Subramaniyam et al. found higher depression scores using the Zung Self-Rating Depression Scale in patients who experienced any shock, including PS, compared with those with no shocks (p=0.048).22 Amiaz et al. reported significant differences in depressive scores using MINI and the Hamilton Anxiety Rating Scale between the PSs and no PSs groups (p=0.058).23 Bilanovic et al. found higher depression scores in the PSs group using HADS, with a p-value of 0.176, an F-value of 2.105 and a ηp² of 0.118, indicating a medium association, although they noted the study was underpowered.20 Varghese et al. reported higher HADS scores in the PSs group at follow-up (p=0.06), and known depression in 7.4% of the PSs group versus 7.1% in the no PSs group.17
All of the previous studies investigated depression after the PS. However, Jacob et al. studied patients with previously confirmed clinical depression and found that 31.6% of patients with PS had depression, compared with 7.9% of patients without PS.16 This difference was statistically significant (OR 8.292; 95% CI [1.781–38.599]; p=0.001). This study was the first to consider pre-existing depression, indicating that PS patients have a higher prevalence of depression. In contrast, Starrenburg et al. did not find a significant relationship between PSs and depressive symptoms using the HADS and MINI scores (p=0.368).21
Relationship of Phantom Shocks to Higher Education
Two studies examined the relationship between education level and PS occurrence. Varghese et al. found a predictive relationship that individuals with higher education levels, specifically those with a bachelor’s degree or higher, had a significantly higher likelihood of PSs (OR 2.8; 95% CI [1.2–6.2]; p=0.03).17 In contrast, Prudente et al. found no significant difference in years of education between PS, the actual shock group and the no shock group. The difference was not statistically significant (p=0.45).14
Relationship of Phantom Shocks to Other Factors
In all the studies, there was no association between patients’ characteristics and PSs. According to Varghese et al., there is a link between PSs and non-ischaemic cardiomyopathy (OR 3.0; 95% CI [1.3–6.7]; p=0.007).17 Also, their study found that a longer duration since device implantation was linked to a higher occurrence of PSs. Specifically, patients who experienced PSs had a device implantation duration of 105 ± 59 months, compared with those without PSs, who had a duration of 61 ± 42 months. This difference was statistically significant, with a p-value of <0.0001. In another study by Kraaier et al., it was found that PSs were linked to a history of AF (p=0.03) and New York Heart Association (NYHA) class <III (p=0.05) in the primary prevention group.18 Jacob et al. found that PS patients had a higher prevalence of documented cocaine use (42.1%).16
Discussion
Extensive research has focused on ICDs, but there is still a need for documentation on PSs. It is crucial to gain further insights into the frequency, predictors, risk factors, management and health implications of PSs. We have thoroughly reviewed the available data and identified cohorts for future guidance to assist patients with this phenomenon. PSs are not uncommon, with prevalence estimates ranging from 2% to 25% in various studies (Table 1). However, these rates could be underestimated, as some PSs may not have been adequately recorded. It is crucial to identify and provide reassurance and appropriate treatment to patients exhibiting these symptoms to reduce their negative impact. Identifying factors that may predispose patients to these conditions is important for identifying those at higher risk.
Out of the studies reviewed, some found a correlation between actual ICD shocks and PSs. Furthermore, one study linked PSs with DFT testing, even though DFT testing is no longer a common practice. In addition, while one study suggested a link between PSs and higher education levels, another study did not find this correlation, indicating that other factors may be more influential. Strangely, none of the studies found a correlation between PSs and patients’ demographics.
Depression has been identified as one important factor, consistently predicting PSs in patients with ICDs. Most importantly, a study by Jacob et al. found that patients with PSs had a higher rate of pre-existing depression than those without PSs (31.6% versus 7.9%).16 That study revealed an OR of 8.292 (95% CI [1.781–38.599]) in favour of depression contributing to PSs.
Anxiety is a significant risk factor for PSs in patients with ICDs. Research shows that anxiety can predict the occurrence of PSs. Patients who have experienced PSs tend to have higher anxiety scores. Psychological assessments can help identify at-risk individuals and tailor interventions accordingly. Patients who experienced PSs had significantly higher HADS scores (15 ± 5.7 versus 8.8 ± 7.4 points; p<0.0001).17
During our review, we encountered an issue of inconsistency in the methodology used across the studies. Combining the results and carrying out a uniform analysis made it difficult. This heterogeneity is present in the assessment tools and statistical methodology. Numerous factors have been examined in relation to PSs. While depression, anxiety, actual shock and DFT testing are considered the most significant, several other associations have been explored in different studies. These include post-traumatic stress disorder, NYHA class less than III, AF, cocaine use, longer duration since device implantation and non-ischaemic aetiology.
The only article that provides an explanatory hypothesis for the pathophysiological understanding of PSs suggests that it may be a recollection of a previously experienced traumatic ICD shock or a distressful memory from shocks delivered during DFT testing or upper limit of vulnerability testing, similar to re-experiencing traumatic events in post-traumatic stress disorder patients.24 This explanation aligns with the positive association found in our review. A perceived ICD shock can initiate a ‘phantom arc’, causing fear and memory consolidation in the amygdala. This negative influence is relayed to the amygdala–hippocampal connections and prefrontal cortex via the thalamus, leading to awareness and future anxiety about the ICD shock. Heightened amygdala response during stress can lead to learning new sensitivities. Memory consolidation of the traumatic event may occur through repetitive stimulation, lowering the threshold for triggering and causing spontaneous firing.16
It is important to inform patients that psychological symptoms can occur. A study by Varghese et al. revealed a concerning finding: patients who experienced PS often felt they did not receive enough information before device placement (22.2% versus 5.0%; p=0.004) and needed psychological support after implantation (25.9% versus 3.3%; p<0.0001).17 This emphasises the need to ensure that patients receive comprehensive and clear information before undergoing procedures. This can help reduce anxiety and better prepare them for potential complications.
There appears to be insufficient focus on the occurrence of PSs in randomised trials. It is also worrisome that quality-of-life studies may need to address this issue satisfactorily. Unfortunately, there is no specific treatment available for PSs. However, standard anxiety and depression treatments can be helpful. Effective options include antidepressants, anti-anxiety medications or support groups.
Conclusion
The occurrence of PSs can be attributed to various factors, including DFT testing, previous ICD shock, depression and anxiety. It is crucial to carefully consider the patient’s condition before ICD implantation, providing needed support and explaining the possibility of PS.