Atrial Fibrillation(AF) is the most common cardiac arrhythmia, with the highest prevalence in elderly patients, and is characterised by an irregular heart rhythm that may result in clots in the heart that can spread throughout the circulatory system. It is seen in approximately 2 % of the European adult population and is a significant cause of increasing healthcare costs in developed countries.1 Its frequency is projected to more than double by mid-century, reflecting the growing proportion of elderly individuals.2 AF influences quality of life significantly and is associated with permanent disability, cognitive disturbance, hospitalisation and absence from work, as well as a five-fold increased incidence of stroke.3 Up to one-quarter of all strokes are attributable to documented AF.4 The aims of AF management are therefore two-fold: rate/rhythm control and anticoagulation.
In patients with AF, aspirin reduces stroke by 22 % compared with placebo. However, patients treated with aspirin have an increased bleeding risk similar to that of vitamin K antagonist (VKA)-treated patients.5,6 The use of aspirin for stroke prevention has been superseded by VKAs, and aspirin is not recommended for this indication.7 The efficacy of oral anticoagulant (OAC) therapy for stroke prevention in AF (SPAF) has been well-established;8 anticoagulation using VKAs such as warfarin has been the mainstay of AF treatment for many years.9 A metaanalysis of six placebo-controlled trials showed that warfarin significantly reduced stroke risk by 64 %.10 However, in the last decade, the limitations of VKAs have led to the development of non-VKA oral anticoagulants (NOACs), including dabigatran11 (a direct thrombin inhibitor), apixaban,12 rivaroxaban13 and edoxaban14 (factor Xa inhibitors). In clinical trials, all NOACs have proved non-inferiority, and some superiority to warfarin in terms of stroke prevention and bleeding risk.11–14 Several guidelines worldwide recommend the use of OACs in patients with AF and ≥1 additional risk factor for stroke (for a risk score of 1, the European and US guidelines issue a Class IIa recommendation, whereas the Canadian guideline issues a Class IIb recommendation).7,15,16 However, the benefit–risk ratio of OAC therapy, i.e. the net benefit of risk reduction of embolic ischaemic events versus the increased risk for bleeding, should be assessed on a case-by-case basis. This enables therapy to be tailored to the specific requirements and risk factors of an individual. This article will discuss individualising anticoagulant therapy in AF patients.
Assessment of Stroke Risk
Various reviews have identified the most consistent independent risk factors for AF-related stroke.17,18 However, risk stratification schemes based on these risk factors have modest predictive value for identifying high-risk patients,18 therefore the focus has shifted towards identifying low-risk patients who do not need anticoagulation therapy.7 Across international guidelines, the CHA2DS2-VASc risk score (congestive heart failure [e.g. left ventricular ejection fraction (LVEF) <40 %], hypertension, diabetes, vascular disease [e.g. prior myocardial infarction (MI), peripheral arterial disease (PAD)], age 65–75, female sex all 1 point; age ≥75, stroke/transient ischaemic attack (TIA) each 2 points) is used to identify low-risk patients, i.e. a CHA2DS2-VASc score of 0 for men or 1 for women, for whom the absolute risk of stroke is less than 1 % per year.19 A 2011 model showed the threshold for ischaemic stroke above which OAC therapy should be considered to be >0.9 %/year for NOAC therapy and >1.7 %/year for warfarin therapy.20
Recently, the stroke risk of patients with a CHA2DS2-VASc score of 1 has become the focus of discussion. Among different studies, annual stroke risk for AF patients with a CHA2DS2-VASc score of 1 not on OAC therapy, varies from 0.6 % to >2.0 %. In a large retrospective study of Swedish health registries of AF patients with a CHA2DS2-VASc score of 1, and who were not exposed to OAC therapy at any time during follow up, the annual ischaemic stroke risk was 0.1–0.2 % for women and 0.5–0.7 % for men. Using a wider definition of ischaemic embolic events, including TIA and pulmonary embolism, the annual event rate was 1.3 % in men.21 In a large retrospective study of Danish Health registries of AF patients, in untreated patients with a CHA2DS2-VASc score of 0 (male) or 1 (female), the annual stroke event rate was 0.49 %. In this study, the annual stroke risk increased to 1.55 % in patients with one additional risk factor.22
There has been debate as to whether in all patients a CHA2DS2-VASc score of 1 supersedes the threshold for ischaemic stroke above which OAC therapy should be considered.22–24 However, not all risk factors in the CHA2DS2-VASc score carry an equal risk. Higher age is the risk factor associated with the highest stroke risk. In a large Taiwanese population-based study of AF patients with CHA2DS2-VASc score of 1 (male) or 2 (female) and not receiving anticoagulation therapy, ischaemic stroke risk in men ranged from 1.96 %/year for men with a CHA2DS2-VASc score of 1 based on the presence of vascular disease to 3.50 %/year for men with a CHA2DS2-VASc score of 1 based on an age of 65–74 years. Ischaemic stroke risk in women ranged from 1.91 %/year for women with a CHA2DS2-VASc score of 2 based on the presence of hypertension to 3.34 %/year for women with a CHA2DS2- VASc score of 2 based on an age of 65–74 years.24
Based on these data, in line with international guidelines, oral anticoagulation should be considered in AF patients with one additional stroke risk factor.7,15,16,24
Assessment of Bleeding Risk
Bleeding is the most feared complication of OAC therapy. Warfarinrelated bleeding is responsible for one-third of all hospitalisations for adverse drug events.25 Most risk factors for stroke are also risk factors for bleeding. Even though AF patients with a higher bleeding risk have a greater net clinical benefit with OAC therapy (the absolute reduction in stroke risk outweighs the absolute increase in bleeding events),5 bleeding risk is the most important barrier to initiating OAC therapy.26 Three bleeding risk scores have been validated in AF populations: the HEMORR2HAGES, HAS-BLED and ATRIA risk scores.27–29 However, only HAS-BLED has been validated in large real-world populations and offers better prediction accuracy than the others.30 Bleeding risk assessment using the HAS-BLED risk score is therefore recommended for all patients with AF.7,31 The HAS-BLED risk score assigns 1 point for each of the following: hypertension (>160 mmHg); abnormal renal/ liver function; previous stroke; bleeding history or predisposition, labile international normalised ratio (INR), elderly, concomitant drugs/ alcohol excess. HAS-BLED scores ≥3 indicate a high risk of bleeding.28 The HAS-BLED score should not be used to exclude patients from OAC therapy, but to address modifiable bleeding risks.32
In summary, initiation of OAC therapy should be based on the individual assessment of stroke risk. According to the European Society of Cardiology (ESC) guidelines, in AF patients with a CHA2DS2- VASc score of 0 in males or 1 in females (annual stroke risk <1 %/year), initiation of OAC therapy is not recommended. In AF patients with one additional CHA2DS2-VASc risk, OAC may be considered. Regardless of the HAS-BLED score, in AF patients with CHA2DS2-VASc score ≥2 there is an obvious net clinical benefit for OAC therapy.7
Non-vitamin K Antagonist Oral Anticoagulants: Pharmacokinetics and Clinical Trial Design
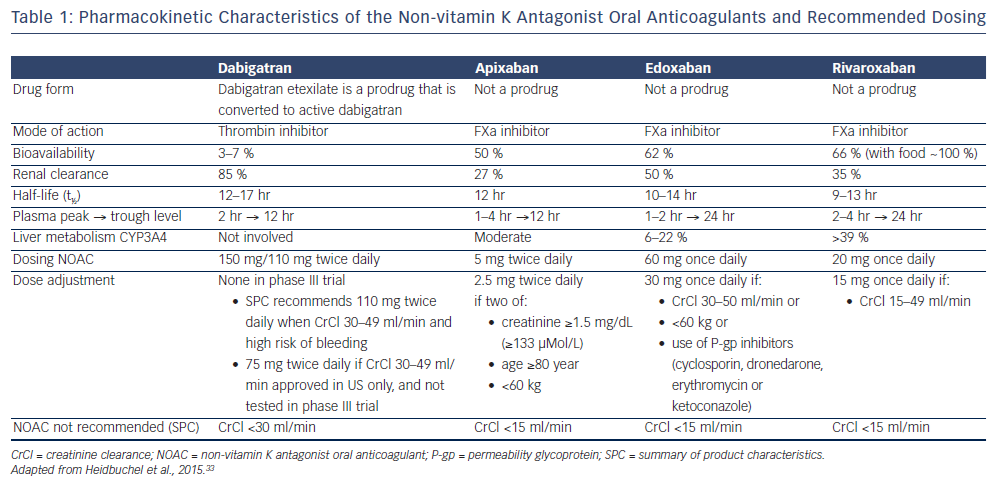
All NOACs share a relative short elimination half-life of approximately 12 hours. Edoxaban and rivaroxaban are administered once daily, dabigatran and apixaban twice daily (see Table 1). Renal clearance shows marked differences among the NOACs, ranging from 27 to 50 % for the factor Xa (FXa) inhibitors to 85 % for dabigatran.11–14,33 NOACs exhibit little potential for drug-drug interactions, and do not require dose adjustment on the basis of coagulation tests like VKAs. However, dose adjustment may be indicated depending on clinical characteristics of the patient and use of concomitant medication.
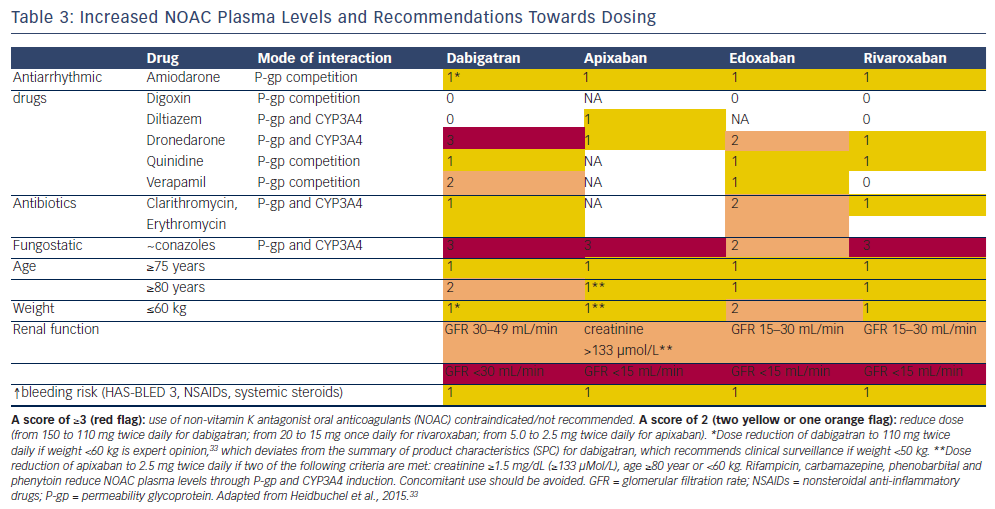
Intestinal absorption of NOACs is dependent on a permeability glycoprotein (P-gp) transporter. Therefore, co-medication that is competing with the P-gp transporter will increase NOAC plasma levels. P-gp inhibitors commonly used in AF patients include verapamil, dronedarone and quinidine. As dabigatran has a much lower bioavailability than the other NOACs (approximately 7 % versus ≥50 %), P-gp inhibitors will especially affect dabigatran levels. Conversely, P-gp inducers such as rifampicin will reduce the NOAC plasma level. Cytochrome P450 (CYP3A4) is involved in the hepatic clearance of rivaroxaban and, to a lesser extent, of apixaban and edoxaban, but not of dabigatran. Strong CYP3A4 inducers or inhibitors have the potential to influence their plasma levels, especially rivaroxaban levels. However, these do not require routine monitoring. In real-life settings, in patients with a high bleeding risk, dose reduction of NOACs has been suggested when higher plasma levels can be expected.
The European Heart Rhythm Association (EHRA) has published a practical guide to the use of NOACs.33 This provides recommendations on: initiating therapy; monitoring the anticoagulant effect; drugdrug interactions; switching between anticoagulant regimens; ensuring compliance; dealing with dosing errors; managing bleeding complications; and special indications in patients undergoing surgical interventions or cardioversion; as well as patients with chronic kidney disease, acute stroke, coronary artery disease and malignancies.33
Phase III Clinical Trials
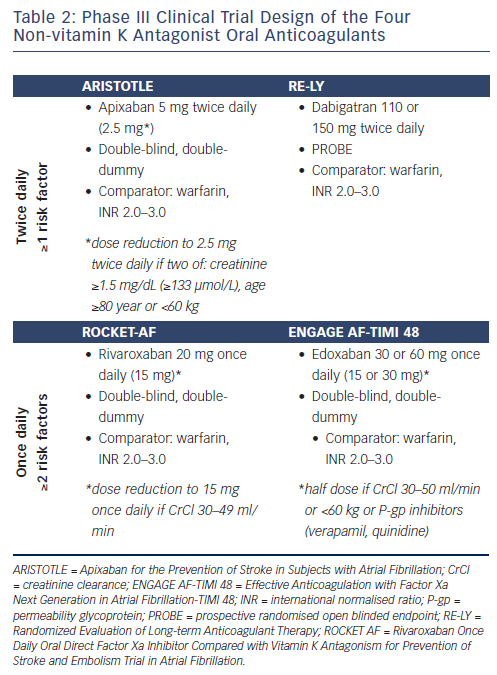
The efficacy and safety of NOACs for SPAF have been established in four pivotal phase III trials. Dabigatran, apixaban, rivaroxaban and edoxaban were studied in the Randomized Evaluation of Long-term Anticoagulant Therapy (RE-LY) trial, the Apixaban for the Prevention of Stroke in Subjects with Atrial Fibrillation (ARISTOTLE) trial, the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibitor Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET AF) and the Effective Anticoagulation with Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48 (ENGAGE AF-TIMI 48) trial, respectively.11–14 Among these clinical trials, distinct similarities and differences can be discerned (see Table 1 and 2).
In all of the phase III NOAC trials in patients with AF, dose-adjusted warfarin with a target INR of 2–3 was used as the comparator arm (see Table 1 and 2). The primary endpoint in all of these trials was the reduction in stroke and systemic embolism,11–14 and the safety endpoint was reduction in major bleeding according to the definition of the International Society on Thrombosis and Haemostasis (ISTH).11–14 However, in ROCKET AF, the principal safety endpoint was a composite of major and non-major clinically relevant bleeding events.13 In the RE-LY trial, a prospective randomised open blinded endpoint (PROBE) design was used. In contrast, in the FXa inhibitor studies a double-blind, double-dummy design was used. A non-inferiority design was used in all studies, with hierarchical statistical testing for superiority once the non-inferiority margin was met. The choice for non-inferiority designs is based on the proven efficacy of warfarin in SPAF. The non-inferiority boundary, which is set to ensure that the study drug preserves a pre-specified portion of the benefit of warfarin over placebo, was set between <1.38 and <1.46, i.e. the study drug would be declared non-inferior if the confidence interval excluded that the primary outcome rate with study drug was >1.38 to >1.46 times higher than with warfarin.11–14 Once the non-inferiority criterion was satisfied, superiority for the primary efficacy endpoint could be tested.
In the RE-LY trial, two different doses of dabigatran, 110 mg or 150 mg twice daily, were evaluated, without dose reduction.11 In the ARISTOTLE trial, apixaban 5 mg twice daily was used, with dose reduction to 2.5 mg twice daily for subjects who at baseline fulfilled two out of three criteria (age ≥80 years, body weight ≤60 kg and serum creatinine level ≥1.5 mg/dL [133 μmol/L]).12 In the ROCKET AF, rivaroxaban 20 mg Once daily was tested, with dose reduction to 15 mg Once daily for subjects with a reduced creatinine clearance (CrCl) of 30–49 mL/min.13 In the ENGAGE AF-TIMI 48 trial, two doses of edoxaban, 30 mg or 60 mg Once daily, were evaluated, with a 50 % dose reduction to 15 mg or 30 mg Once daily, respectively, for subjects with a CrCl 30–50 mL/min, a body weight ≤60 kg, or concomitant administration of verapamil, quinidine or dronedarone (strong P-gp inhibitors).14
Patients with AF and ≥1 additional stroke risk factor were included in the RE-LY and the ARISTOTLE trials, and with ≥2 additional stroke risk factors in the ROCKET-AF and the ENGAGE AF-TIMI 48 trial, i.e. patients in the ROCKET-AF and the ENGAGE AF-TIMI 48 trial were at higher risk of stroke compared with the patient populations in the RE-LY and ARISTOTLE trials. Consequently, the underlying risk for stroke differed significantly across the trials, with mean CHADS2 scores ranging from 2.1 to 3.5.11–14
In summary, when interpreting efficacy and safety data, it is important to realise that there are distinct differences between these phase III NOAC trials. Given the heterogeneity of the different trials and in the absence of head-to-head studies, a comparative efficacy of the individual four NOACs cannot be assessed. In a meta-analysis of the four phase III trials, compared with warfarin, NOACs significantly reduced the risk of stroke (HR 0.81; 95 % CI 0.73–0.91), intracranial haemorrhage (HR 0.48; 95 % CI 0.39–0.59) and mortality, with a similar risk for major bleeding (HR 0.86; 95 % CI 0.73–1.00), but increased gastrointestinal bleeding (HR 1.25; 95 % CI 1.01–1.55).34 The 19 % risk reduction in stroke or systemic embolism was mainly driven by a 51 % reduction in haemorrhagic stroke (HR 0.49; 95 % CI 0.38–0.64). NOACs were similar to warfarin in prevention of ischaemic stroke (HR 0.92; 95 % CI 0.83–1.02) and myocardial infarction (0.97; 95 % CI 0.78–1.20). The benefits of reduction in stroke or systemic embolism and reduction in bleeding were consistent across multiple subgroups examined, with no interaction for age, CrCl and time in therapeutic range for warfarin.34
Based on their favourable benefit–risk ratio, regulatory agencies have approved the use of NOACs for reduction of risk of stroke in patients with non-valvular AF. Furthermore, international guidelines on the treatment of AF have now issued a class I recommendation for the use of NOACs for stroke prevention in patients with AF and a CHA2DS2- VASc score ≥2,7,15 and a class IIa recommendation for the preferred use of NOACs over VKA.7,16
Real World Effectiveness Data of Non-vitamin K Antagonist Oral Anticoagulants
The efficacy of NOACs has been established within the setting of well-controlled phase III trials, with strict inclusion and exclusion criteria, and control of therapy adherence and use of concomitant medication. However, the safety profile of NOACs demonstrated in controlled trial settings may be different in a real-life setting. The comparative effectiveness of dabigatran versus warfarin was studied in a large cohort of 134,414 propensity score-matched Medicare beneficiaries. Compared with warfarin, dabigatran reduced the risk of ischaemic stroke (HR 0.80 [0.67–0.96]), intracranial haemorrhage (HR 0.34 [0.26–0.46]) and death (HR 0.86 [0.77–0.96]); there were no differences between cohorts in risk of major bleeding (HR 0.97 (0.88–1.07) or acute MI (HR 0.92 [0.78–1.08]), and there was an increased risk of gastrointestinal bleeding (HR 1.28 [1.14–1.44]).35 In the RE-LY trial, patients were randomised to dabigatran 150 mg twice daily or 110 mg twice daily. In this US cohort study, however, 16 % of the patients used dabigatran 75 mg twice daily. In the subgroup treated with dabigatran 150 mg twice daily, the magnitude of effect for the above reported outcomes was greater. In the subgroup treated with dabigatran 110 mg twice daily, none of the outcome comparisons were statistically significantly different from warfarin except for a lower risk of bleeding.
In a prospective, non-interventional oral anticoagulation registry of 1,776 daily-care patients (Dresden NOAC registry), rivaroxaban-related major bleeding (3.1 %/year) compared well to major bleeding in the ROCKET-AF (3.4 %).36 In an observational pharmacovigilance study of 27,467 rivaroxaban users, incidence of major bleeding was 2.86 %/ year, consistent with the phase III trial results.37 To date, no real-world registry data are yet available for apixaban and edoxaban. Data for edoxaban are currently being accrued through the Edoxaban Treatment in Routine Clinical Practice – Atrial Fibrillation – Europe (ETNA-AF-Europe) registry, which recently commenced enrolment and aims to recruit approximately 13,000 patients from 1,450 sites across 12 countries.38
Despite the more favourable safety profile of NOACs, the initial absence of specific antidotes to reverse the anticoagulant effect of NOACs may, rightly or wrongly, have formed an obstacle to their use in daily care. For rapid reversal of life-threatening warfarin-associated bleeding, in addition to general measures such as discontinuation of the anticoagulant and supportive measurements, the administration of four-factor prothrombin complex concentrates (PCC) is recommended, together with vitamin K to allow for de novo synthesis of coagulation factors.39 However, despite rapid normalisation of the INR, the prognosis of warfarin-associated major bleeding remains poor. For rapid reversal of life-threatening NOAC-associated bleeding, the administration of PCC may be considered in addition to general measures.33
Recently, data on specific reversal agents have been published. Idarucizumab, a monoclonal antibody fragment, was designed to specifically reverse the anticoagulant effect of dabigatran. The Reversal Effects of Idarucizumab on Active Dabigatran (REVERSEAD) trial included patients with serious bleeding or those requiring urgent surgical procedure. An interim analysis after 90 patients were recruited showed that 5 g IV idarucizumab completely reversed the anticoagulant effect of dabigatran within minutes.40 The trial, however, was not designed to compare clinical outcome data. Based on these data, in patients presenting with dabigatran-associated lifethreatening bleeding, idarucizumab, a monoclonal antibody fragment, is the preferred reversal agent33 and has been shown to be safe in initial clinical trials.41,42 Andexanet alfa, a recombinant modified human factor Xa decoy protein, was designed to specifically reverse the anticoagulant effects of factor Xa inhibitors. In the Andexanet Alfa for the Reversal of Factor Xa Inhibitor Activity (ANNEXA) trials, 101 healthy older volunteers were given either apixaban or rivaroxaban and then randomised to andexanet or placebo. Administration of an andexanet bolus followed by a 2-hour infusion of andexanet reduced anti-FXa activity by 92 % and 94 %, respectively.43 The availability of NOACspecific antidotes may take away possible concerns about reversal of NOAC activity, but these agents should be reserved for life-threatening bleeding or for urgent surgical procedures or thrombolysis. A detailed description of the management of bleeding complications can be found in Heidbuchel et al.33
In conclusion, the effectiveness of two NOACs in real world registry studies compares well to their phase III clinical trial results. More observational data on real life effectiveness of NOACs will emerge from on-going large registries with all four NOACs.44
Individual Factors Affecting Treatment Choice
Since the introduction of NOACs, a range of questions relating to their use has emerged, which may affect treatment choice.45,46 There are no published trials comparing NOACs head-to-head, and it is unlikely that any will be conducted in the near future. It is essential to select the appropriate patients for treatment with NOACs to reduce the risk of adverse events and ensure optimal outcomes. A comprehensive guide on the practical use of NOACs has been published by Heidbuchel et al.,33 and is available online at www.NOACforAF.eu. A thorough patient history should be taken, including concomitant prescription and over-the-counter medications and assessment of kidney function. Dose reduction may be indicated in at-risk patient populations:
- For dabigatran, it is recommended to reduce the dosage to 110 mg twice daily in patients ≥80 years or in patients receiving concomitant verapamil. In patients aged 75–80 years and in patients with moderate renal impairment, dose reduction may be considered according to physician discretion in patients at high bleeding risk.47
- For apixaban, it is recommended to halve the dosage to 2.5 mg twice daily in patients with at least two of the following characteristics: age ≥80 years, body weight ≤60 kg or serum creatinine ≥1.5 mg/dL.48
- For rivaroxaban, dose adjustment to 15 mg once daily is recommended in patients with moderate to severe renal impairment (CrCl 15–49 mL/min), but no dose adjustment is recommended for body weight or elderly age.49
- For edoxaban, dose adjustment to 30 mg once daily is recommended in patients with moderate to severe renal impairment (CrCl 15–50 mL/min), and/or body weight ≤60 kg and/or concomitant use of strong P-gp inhibitors (cyclosporin, dronedarone, erythromycin, ketoconazole; see Table 3).50
Adherence to Dosing Regimen
As a first step, patients taking NOACs should be well-informed about the importance of adherence to the scheduled dosing regimen. Depending on the patient’s preference, once daily or twice daily dosing may be chosen. Medication possession ratio (MPR; number of days of medication supplied between first prescription and subsequent 365 days, divided by 365) is used as a surrogate parameter for medication intake. In a retrospective Medicare cohort of 1,440,254 patients taking an antidiabetic, antihyperlipidemic, antiplatelet, or cardiac agent with once daily or twice daily dosing, compared with twice daily, once daily dosing increased overall MPR from 57 % to 66 %, respectively (+15.8 %). However, no difference was seen in the MPR for cardiac agents (0.63 in both groups).51 In a retrospective cohort study of 10,697 patients with AF initiated on oral once daily or twice daily regimens of antidiabetic or antihypertensive medications, the MPR was 75.3 % and 70.4 % for once daily and twice daily regimens, respectively. Notably, adherence as represented by the MPR was highest in patients >65 years of age, with a similar MPR for once daily dosing versus twice daily dosing (87 versus 85 %).52 In a Danish cohort study of 2,960 AF patients using dabigatran twice daily, after one year the overall proportion of days covered (PDC; sum of days of supply of medication, divided by the number of days evaluated) was 83.9 %, with 76.8 % of the patients having a one year PDC >80 %. Patients with increased risk for stroke (CHA2DS2-VASc scores ≥2), regular users of cardiovascular drugs and patients with a history of stroke/TIA all showed more adherence than the reference group with a CHA2DS2-VASc score of 1.53
There is considerable debate about the benefits of once daily administration over twice daily. Several studies suggest that once daily dosing may increase adherence.44,46–48 In two studies of insurance claims database (n=10,697 and 16,014, respectively), patients with AF treated with once daily dosing regimens for long-term medications were associated with significantly higher adherence compared with subjects on twice daily regimens.52,54 A systematic review and meta-analysis of four randomised control trials (a total of 2,557 patients) involving patients with chronic cardiovascular disease found a significant 56 % reduction in the risk of non-adherence to drug therapy.55 However, there is a disproportionately greater impact on drug action of missing a dose of a once daily than a twice daily NOAC.56
Concomitant Medications
Assessment of concomitant medication is an important consideration when initiating OAC therapy. The elderly AF population often uses many concomitant medications. For example, in the ROCKET AF, at baseline, 51 % of patients were on 5–9 concomitant medications and 13 % were on ≥10 concomitant medications.57
To assist with self-administration and compliance with multiple dosage instructions, dispensed drugs may be repackaged into smaller, ready to dispense quantities from larger bulk containers. Dabigatran capsules, however, can only be dispensed and stored in the manufacturer’s original packaging to protect from moisture, and therefore are not suitable for repackaging.47 The EHRA has proposed that patients taking NOACs carry a patient information card that provides information both for the patient (instructions on correct intake) and healthcare workers (renal function, concomitant medication, etc.).33
Over-the-counter drugs such as nonsteroidal anti-inflammatory drugs (NSAIDs), antiplatelet therapy (APT) or drugs that compete with the P-gp transporter (e.g. verapamil) and/or CYP3A4 (like fungostatics) can increase bleeding risk.58 The occurrence of drug-drug interactions should be avoided as much as possible, and not be used as an argument to refrain from OAC therapy. If higher NOAC plasma levels are expected, dose reduction should be considered. For an extensive overview of drug-drug interaction and recommendations for dose reduction, see Heidbuchel et al.33
Patients with AF receiving oral anticoagulants are often treated with concomitant APT, mostly aspirin, even in the absence of cardiovascular disease. However, concomitant use of aspirin and warfarin significantly increases the risk for major bleeding.59
In the RE-LY trial, 38.4 % of the enrolled patients received concomitant APT at some time during the study. Dabigatran 110 mg twice daily was non-inferior to warfarin in reducing stroke, without an interaction for APT (with APT: HR 0.93; [0.70–1.25]; without APT: HR, 0.87 [0.66–1.15]).60 Dabigatran 150 mg twice daily was superior to warfarin in reducing stroke in the overall patient population; however, this effect seemed attenuated among patients who used APT (with APT: HR 0.80 [0.59–1.08], without APT: HR 0.52 [0.38–0.72]). Concomitant use of APT increased the risk of major bleeding; however, the relative advantage of dabigatran over warfarin in reducing major bleeds showed no interaction for aspirin use.60 In the ARISTOTLE trial, 24 % of the enrolled patients were using aspirin at baseline. The benefits of apixaban in stroke prevention (with aspirin: apixaban 1.12 %/year versus warfarin 1.91 %/year, HR 0.58 [0.39–0.85] versus without aspirin: apixaban 1.11 %/year versus warfarin 1.32 %/year, HR 0.84 [0.66–1.07]) and the reduction in major bleeding (with aspirin: apixaban 3.10 %/year versus warfarin 3.92 %/ year, HR 0.77 [0.60–0.99] versus without aspirin: apixaban 1.82 %/year versus warfarin 2.78 %/year, HR 0.65, [0.55–0.78]) showed no interaction for aspirin use.61 In the ROCKET AF, 35 % of the enrolled patients were using concomitant aspirin therapy. Aspirin use was associated with increased risk of major bleeding (with aspirin use: rivaroxaban 4.52 %/ year versus warfarin 4.12 %/year, without aspirin use: rivaroxaban 3.11 %/year versus warfarin 3.11 %/year).13 Rivaroxaban was noninferior to warfarin for the occurrence of major bleed, without an interaction for APT use (with aspirin use: HR 1.10 (0.89–1.36), without aspirin use: HR 1.0 (0.83–1.20). In the ENGAGE AF-TIMI 48 trial, 23 % of the enrolled patients were using concomitant APT, mostly aspirin, at 3 months. Relative efficacy in stroke prevention and reduction in major bleeding showed no interaction with concomitant antiplatelet use.62,63
In conclusion, concomitant use of antiplatelet drugs increases the absolute risk for major bleeding, without affecting the relative benefits of NOACs over warfarin. Recommendations are given in the individual summary of product characteristics (SPC) for the administration of NOACs with APT and/or aspirin.
Age
Approximately 15 % of patients with AF are <60 years of age, whereas more than one-third of patients with AF are ≥75 years of age.2 The risk of stroke and the risk of major bleeding increases with age. All anticoagulants will cause more bleedings in elderly patients, especially in the presence of other bleeding risk factors. In the RE-LY trial, 7,258 patients were ≥75 years of age. For the primary efficacy outcome of stroke or systemic embolism, the benefit of dabigatran versus warfarin was independent of age. The risk of major bleeding increased with age. With increasing age, the benefit in reducing major bleedings was attenuated with similar and higher bleeding rates compared with warfarin for dabigatran 110 mg twice daily and 150 mg twice daily, respectively.64 In a pre-specified secondary analysis of the ARISTOTLE trial, patients were stratified into age categories: <65 (n=5,471), 65–74 (n=7,052) and ≥75 (n=5,678) years. The risk of major bleeding and the risk of stroke/systemic embolic events (SEE) increased with age. The benefits of apixaban in stroke prevention showed no interaction with age. Apixaban reduced the rate of major bleeding compared with warfarin with a consistent treatment effect across age groups.65 In a pre-specified secondary analysis of the ROCKET-AF, patients were stratified into age categories: <75 (n=8,021) and ≥75 (n=6,215) years. The risk of major bleeding and the risk of stroke/SEE increased with age. The efficacy of rivaroxaban in stroke prevention and the occurrence of bleeding showed a consistent treatment effect across age groups.66 In a pre-specified secondary analysis of the ENGAGE AF-TIMI 48 trial, patients were stratified into age categories: <65 (n=5,497), 65–74 (n=7,134) and ≥75 (n=8,474) years. The risk of major bleeding and stroke/SEE increased with age, but more markedly so for major bleeding. The treatment effects of edoxaban seen in the overall patient population were consistent in different age subgroups, with a major impact on absolute risk reduction for major bleeding, without an interaction for age. In addition, the absolute benefits of edoxaban tended to be greater in the elderly.67 These observations were supported in a recent systematic review and meta-analysis of 19 NOAC trials conducted in patients with AF or venous thromboembolism (VTE).68
In summary, in phase III trials, when compared to warfarin, in terms of efficacy and safety the benefits of NOAC therapy are consistent regardless of age. A 2015 ESC consensus document that focused on age-specific risks and benefits of antithrombotic drugs tested in phase III trials, provided recommendations on dose reduction in the elderly.69 For dabigatran a dose reduction to 110 mg twice daily for age 75–79 years is recommended,70 which is the European Medicines Agency (EMA)-approved dose for patients ≥80 years. For apixaban a dose reduction to 2.5 mg twice daily is recommended if age ≥80 years and body weight is 60 kg or serum creatinine is ≥1.5 mg/dL, similar to the EMA approval. No age specific dose adjustments for rivaroxaban and edoxaban are recommended in this consensus document.
Renal Function
Chronic kidney disease (CKD) is common in patients with AF, with a third of patients having stage III CKD (estimated glomerular filtration rate [eGFR] 30–60 mL/min).71 In patients with AF, renal failure is a risk factor for both stroke and bleeding.72 Among NOACs, the renal clearance varies considerably, from 27 % for apixaban48 to 85 % for dabigatran,47 and dose reductions may be indicated in patients with reduced renal function. Therefore, renal function should be monitored yearly, but more frequently in patients ≥75 years of age using dabigatran or edoxaban, i.e. at 6 months intervals.33 Quarterly monitoring of renal function is recommended in patients with CrCl 15–30 ml/min.
Patients with AF and mild-to-moderate renal failure (CrCl 30–50 mL/min) were enrolled in the phase III trials.11–14 As pre-specified per protocol, rivaroxaban, apixaban and edoxaban dosing was reduced in these patients, and dabigatran dosing was not. In the ENGAGE AF-TIMI 48 trial with edoxaban, the dosing was adjusted both at the time of randomisation and also during the trial, to resemble real world clinical practice.14 In a pre-specified secondary analysis of the ENGAGE AF-TIMI 48 trial, patients were stratified into eGFR categories ≤50 and >50 mL/min. The relative risk of stroke/systemic embolism with edoxaban vs warfarin in the pre-specified analysis in those with CrCl ≤50 (HR 0.87, 0.65–1.18) was similar to those with CrCl >50 (HR 0.87, 0.72–1.04; p-interaction = 0.94). While there is a trend towards decreasing efficacy with increasing CrCl for edoxaban compared with well-managed warfarin, the overall safety and net clinical benefit of edoxaban compared to warfarin is consistent across renal function groups.73
In a pre-specified secondary analysis of the ARISTOTLE trial, patients were stratified into eGFR categories: >80 (n=7,518), >50–80 (n=7,587) and ≤50 (n=3,017) mL/min. The risk of stroke and the incidence of major bleeding increased with deteriorating renal function in the overall population irrespective of treatment. The benefits of apixaban in stroke prevention showed no interaction with renal function. The relative reduction in major bleeding with apixaban was significantly greater in patients with an eGFR ≤50 mL/min.74 In a pre-specified secondary analysis of the ROCKET-AF, patients were stratified into eGFR categories: ≥50 mL/min (n=11,277), randomised to rivaroxaban 20 mg or warfarin; and 30–49 mL/min (n=2950), randomized to rivaroxaban 15 mg or warfarin. The risk of stroke and the incidence of major bleeding increased with deteriorating renal function. The efficacy of rivaroxaban in stroke prevention showed no interaction with renal function. Rates of major bleeding were similar for rivaroxaban and warfarin, with no interaction for renal function.75
In a pre-specified secondary analysis of RE-LY, patients were stratified into eGFR categories: ≥80 (n=3,880), >50–80 (n=10,697) and ≤50 (n=3,374) mL/min. Again, the risk of stroke and major bleeding increased with deteriorating renal function in the overall population irrespective of treatment. The benefits of dabigatran in stroke prevention showed no significant interaction with renal function (consistent with the overall trial, a lower risk with high dose dabigatran and similar risk with low dose dabigatran compared with warfarin). There was, however, an interaction between treatment and renal function such that compared to warfarin with either doses of dabigatran the relative reduction in major bleeding was greater in patients with an eGFR ≥80 mL/min.76
These observations were supported in a recent systematic review and meta-analysis of five studies comprising 72,845 AF patients randomised to NOAC of warfarin, in which NOACs had similar efficacy and safety compared to warfarin across different levels of renal function.77
In summary, in patients with AF, renal impairment is associated with higher rates of stroke and bleeding. Compared to warfarin, in terms of efficacy and safety the benefits of NOACs are maintained regardless of renal function.78
Conclusion
In patients with AF and ≥1 stroke risk factor, phase III trials have demonstrated the favourable benefit–risk ratio in SPAF of NOACs compared with VKA. Real life data confirm the effectiveness and safety of NOACs, making NOACs the preferred choice over VKA for SPAF. Older age and CKD increase the risk of bleeding for both NOAC as well as for VKA. The benefits of NOACs, however, are maintained in subgroups of elderly patients and patients with CKD. Due to higher rates of stroke and major bleeding in these subgroups, the absolute benefits of NOACs are even greater in these subgroups. In the absence of head-to-head comparisons, there is no single NOAC that can be recommended above the other NOACs. Rather, the caregiver has the opportunity for personalised care, based on clinical factors such as renal function and age, practical factors such as those recommended by the EHRA guide,33 convenience of and likely adherence to the dosing regimen, potential need for dose reduction (for example, in the presence of CKD) and patient preference. Patient compliance is also an important factor to be taken into account when selecting NOACs.
Concomitant medication may increase the risk of bleeding. Therefore, in line with the SPC of the individual NOACs, dose reduction of NOACs is to be considered in the presence of drugs that can be expected to increase the plasma level of NOACs. Although aspirin may be co-administered with all NOACs, in general to reduce the risk of major bleeding where possible, concomitant use of APT should be avoided.47–50 As the incidence of coronary artery disease and AF ranges between 24 and 46 %,79 management of patients treated with a NOAC and presenting with an acute coronary syndrome may deserve special care.
Finally, patient education and knowledge transfer on SPAF are important tools to increase compliance. Resources for patients are available online with the international cardiology societies (e.g. at the ESC/EHRA website, see: www.afibmatters.org; at the AHA website, see: ’TheAFibFive‘ and myafibexperience.org; and patient information on the website of the AF Association: www.atrialfibrillation.org.uk).80