Cardiac resynchronisation therapy (CRT) is an established treatment for patients with symptomatic heart failure, severe left ventricular (LV) dysfunction and electrical dyssynchrony.1,2 However, even in carefully selected patients, approximately 30% fail to respond, and this has led to the development of alternative pacing strategies to improve patient outcomes.3,4 Conduction system pacing with His bundle pacing (HBP) or left bundle branch area pacing (LBBAP) provides physiological activation using the native conduction system.5 LV endocardial pacing enables access to faster endocardial conduction and site-specific pacing, unlike conventional CRT.6–9 This article will discuss LV endocardial pacing and LBBAP in detail, including the potential benefits and risks of each intervention and future directions.
Transvenous Epicardial CRT
Pacing within areas of myocardial scar is associated with poorer outcomes, whereas targeting areas of latest electrical and mechanical activation leads to improved patient outcomes.10–15 Patient-specific pacing that avoids myocardial scar while targeting areas of latest activation is difficult with epicardial CRT because the pacing location is dependent on the coronary sinus anatomy, and the optimal pacing segment may not be subtended by a coronary vein or may result in phrenic nerve stimulation. Pacing in unfavourable locations will result in inadequate resynchronisation and a suboptimal response.3,16 Furthermore, it is estimated that 8–10% of CRT procedures are unsuccessful due to anatomical constraints, such as failure to cannulate the coronary sinus.17 Therefore, given the limitations of epicardial pacing and the need to improve response rates, the role of alternative pacing strategies has become increasingly important.
Endocardial Pacing
Endocardial pacing offers many advantages over epicardial pacing. It enables access to fast endocardial conduction, shorter path length for impulse conduction, a more physiological activation pattern by spreading from the endocardium to the epicardium, a lower pacing capture threshold and a lower risk of phrenic nerve stimulation.6–9 Endocardial pacing is less arrhythmogenic than epicardial pacing and is less affected by myocardial scar location.18 It is also less likely to result in phrenic nerve stimulation such as in epicardial pacing. The greatest potential benefit of endocardial pacing is the ability to pace anywhere inside the left ventricle, enabling the operator to select the optimal pacing site unrestricted by the coronary sinus anatomy. This is particularly attractive in patients with unfavourable characteristics, such as ischemic cardiomyopathy and transmural myocardial scar.
The haemodynamic changes with endocardial and epicardial pacing have been previously studied. In a study of eight anaesthetised dogs with experimental left bundle branch block (LBBB), endocardial pacing was associated with greater electrical resynchronisation, and increase in LV dP/dtmax and stroke work, compared with epicardial pacing.19 Similarly in a study of 22 dogs, endocardial pacing resulted in better electrical resynchronisation and haemodynamic changes than epicardial pacing.20 Large human studies comparing epicardial and endocardial pacing are lacking but smaller studies demonstrate the predominant benefit of endocardial pacing, which is its ability to access the optimal pacing site.21–24
Delivering Left Ventricular Endocardial Pacing
LV endocardial pacing was initially delivered using leads via an atrial transseptal, transventricular septal or transventricular apical approach. Several case series report their experience with lead-based endocardial pacing but are limited by the study design and a small patient cohort.25 The ALSYNC study was the largest prospectively collected, multicentre registry investigating the feasibility and safety of LV endocardial pacing, enrolling 138 patients with a failed conventional LV lead, suboptimal coronary sinus anatomy or CRT non-response.26 Patients were predominantly men, with non-ischaemic cardiomyopathy, LBBB, broad QRS duration and severely impaired LV systolic function. Successful procedures were achieved in 89% of patients, 82% of patients had freedom from complications related to the lead delivery system, implant procedure or lead, and 3.8% of patients had non-disabling strokes. At 6 months, the New York Heart Association (NYHA) functional class improved in 59% of patients, and 55% had a reduction in LV end-systolic volume (LVESV) ≥15%.26 Although the response rate in this difficult patient group was promising, the main limitations were the significant rate of cerebrovascular accidents, the need for lifelong anticoagulation, and the low rate of optimal lead placement (leads could be placed in the desired location in only 81% of implants).
Leadless LV pacing offers many advantages over lead-based pacing, including a reduced risk of lead-related issues (including infection), no requirement for lifelong anticoagulation, and potentially a greater selection of pacing sites. Leadless LV pacemakers need to be compact to ensure that they do not interfere with anatomical structures within the left ventricle, the endocardial wall, or outflow tract. Longer devices with broad batteries are more likely to collide with intracardiac structures, and therefore, to reduce this risk of collision while maintaining the volume for the battery, devices must be shorter and thicker.27 The current generation of leadless pacemakers used in the right ventricle are predicted to be able to be placed in only a limited number of LV endocardial sites due to their dimensions,27 highlighting the importance of optimal length/device width ratio. Currently, the WiSE-CRT system (EBR Systems), in which the power is supplied from a remote battery, is the only commercially available leadless LV endocardial pacing system, and will be discussed further.
WiSE-CRT System
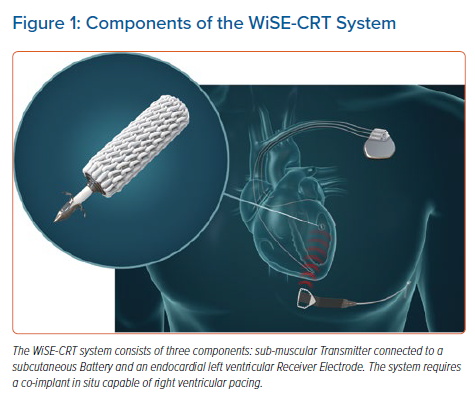
The WiSE-CRT system provides leadless LV endocardial pacing to achieve near simultaneous ventricular activation and resynchronisation. The system consists of three components: a submuscular transmitter, connected to a subcutaneous battery, and an endocardial receiver electrode (Figure 1). The system requires the patient to have a co-implant in situ that is capable of producing continuous right ventricular (RV) pacing. The transmitter and battery detect an RV pacing pulse emitted by the co-implant, and the transmitter emits a number of short ultrasound pulses to locate the electrode. Each pulse is converted into electrical energy to identify the electrode location but is of insufficient magnitude to pace the left ventricle. Once identified, the transmitter sends a focused beam of ultrasound energy to the electrode location and this is converted into electrical energy, causing LV capture and simultaneous biventricular pacing in 2–5 ms. The endocardial electrode can be placed anywhere inside the left ventricle but the energy reaching the electrode reduces with an increased angle and distance between the transmitter and electrode. Patients who have an obtuse angle between devices or increased distance, will have insufficient electrode capture, battery depletion and failure of biventricular pacing. The WiSE-CRT system is indicated in patients suitable for CRT.
Transmitter and Battery Implantation
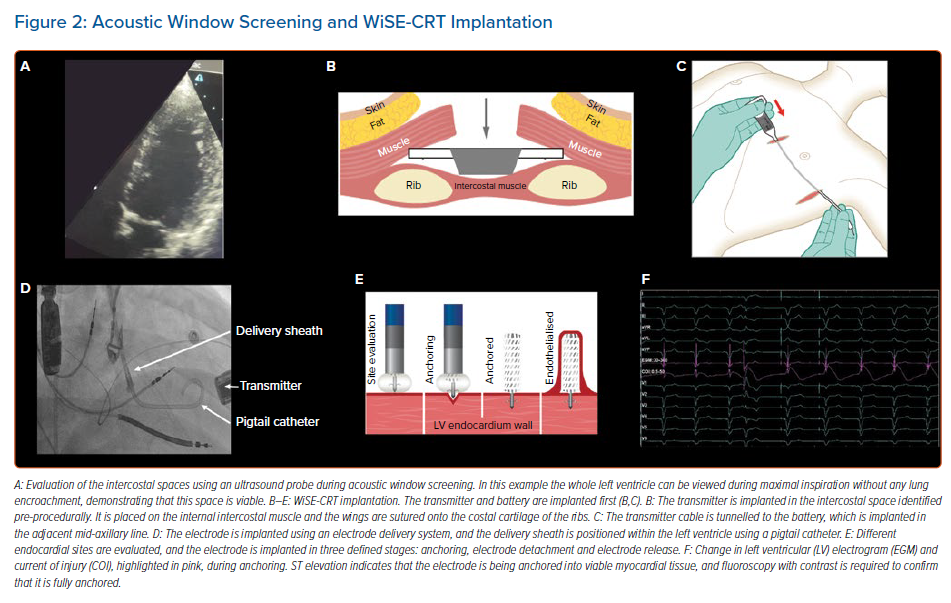
Patients must undergo acoustic window screening to be eligible for the device. This involves placing an ultrasound probe in different intercostal spaces to determine if there is an adequate window. Acceptable windows have no lung encroachment during maximal inspiration (Figure 2) and an angle between the probe and basal posterolateral wall <45°, distance <12 cm and LV wall thickness ≥5 mm. These measurements are repeated with the patient lying supine, on their right side, and while sitting upright. Patients often have more than one intercostal space available for transmitter implantation, enabling the operator to select the optimal site.28
Procedures are predominantly performed under general anaesthesia and can be undertaken in a single-stage or dual-stage procedure, with the latter involving implantation of the battery and transmitter, and the electrode on two separate occasions. The transmitter is always implanted first, is placed on the intercostal muscle and is secured to the costal cartilage, with the battery placed in the adjacent mid-axillary
line (Figure 2). Intra-procedural confirmation of an adequate window to the left ventricle using echocardiography is advised to ensure there is no lung encroachment.
Electrode Implantation
The electrode can be implanted via a retrograde aortic approach using arterial access or a transseptal approach using venous access.29 The electrode delivery system is a catheter-based system used for implanting the electrode, consisting of the electrode and delivery catheter (8 Fr) and a steerable delivery sheath (12 Fr). The delivery sheath has a diameter of 4 mm, therefore confirmation of adequate arterial access is recommended prior to the procedure, and this is possible with CT or ultrasound. Dual femoral arterial access can be used with the aid of an aortogram to ensure that the puncture site for the electrode delivery sheath is correctly sited, to reduce the risk of vascular complications. A trans-oesophageal or intracardiac echocardiogram is performed during electrode implantation to ensure that any complications are identified in a timely manner and to facilitate the implantation of the electrode.
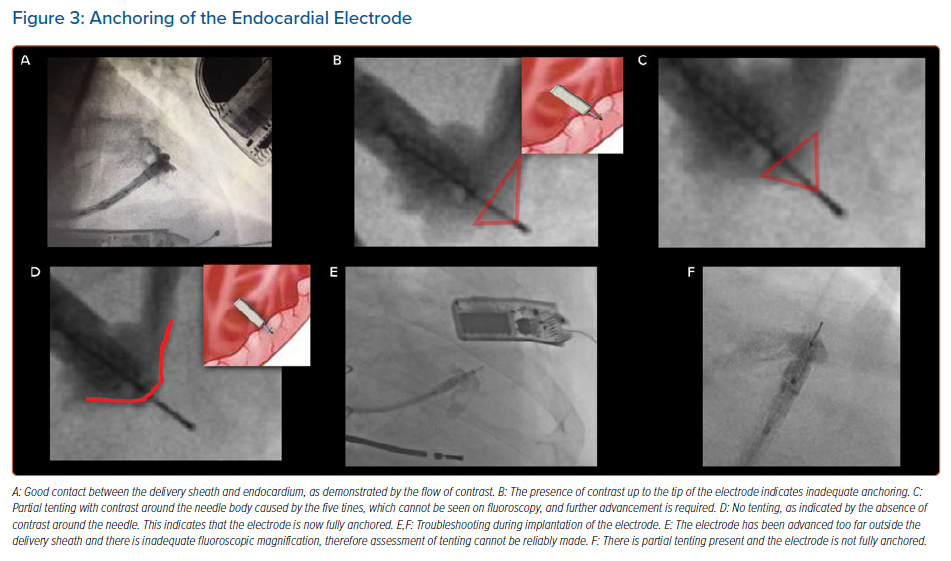
The delivery sheath has a balloon at its distal tip, and once access has been achieved the balloon is inflated. The delivery sheath is positioned within the left ventricle and the electrode catheter is inserted. The delivery sheath is slowly advanced to the desired endocardial location. A tight seal between the balloon and the endocardium is confirmed by a flush of contrast, which should be seen coming around the sides of the balloon rather than forwards (Figure 3). The electrode is implanted in a number of defined stages, as follows:
- Anchoring: a tight seal is maintained between the balloon and the endocardium while the electrode catheter is advanced 1 mm at a time (Figure 3). Simultaneous live fluoroscopy and contrast flushes are used to look for LV tenting. Tenting demonstrates that the electrode tines are still within the cavity of the left ventricle. The electrode is then slowly advanced until there is no tenting, demonstrating that the tines are within the endocardium. The absence of tenting should be confirmed on two orthogonal views, with no contrast beyond the electrode body (Figure 3).
- Electrode detachment: both the delivery sheath and electrode catheter are kept stable and the electrode is detached, resulting in an indicator change on the catheter and a disturbance on the intracardiac electrogram.
- Electrode release: under continuous fluoroscopy, the delivery sheath is slowly retracted until it is aligned with the tip of the catheter; they are then withdrawn together. Satisfactory placement of the electrode can be seen on fluoroscopy, and pacing checks are undertaken to ensure that there is appropriate RV tracking and biventricular pacing.
Outcomes of the WiSE-CRT System
Experience and patient outcomes have been reported in three prospective multicentre trials: the WiSE-CRT study, the SELECT-LV study and the WiCS-LV Post Market Surveillance Registry.30–32 These studies included patients who had a failed LV lead, were considered high-risk for a CRT upgrade or were non-responders to conventional CRT. The WiSE-CRT study was a first-in-man trial, published in 2014, which assessed the feasibility, safety and short-term outcomes of the system in 17 patients.30 That study was stopped early due to a high incidence of pericardial tamponade, occurring in three patients (17.6%). Consequently, the delivery sheath was redesigned to incorporate a balloon at the distal tip to reduce traumatic engagement with the LV endocardium. The feasibility of the WiSE-CRT system using the re-designed delivery sheath was investigated in the SELECT-LV study, involving 35 patients across six centres and was published in 2017.31 The recent publication of the WICS-LV Post Market Surveillance Registry in 2020 determined the safety and efficacy of the WiSE-CRT system in a real-world setting involving 90 patients from 14 European centres.32 The outcomes of the WiSE-CRT system will be discussed further in the following sections using the latter two studies, which have utilised the latest iteration of the redesigned delivery sheath.
Procedural Success
Procedural success was reported in 34 of 35 patients (97.1%) in the SELECT-LV study, given that one patient had a ventricular arrhythmia.31 Successful procedures occurred in 85 of 90 patients (94.4%) in the WICS-LV Post Market Surveillance Registry, with biventricular pacing confirmation after implantation.32 Failure to achieve procedural success was due to failing to exclude unsuitable intercostal spaces during acoustic window screening, pericardial tamponade, transmitter displacement, and implantation of the electrode within suspected myocardial scar.
Response to CRT
Overall at 6 months, 84.8% of patients in the SELECT-LV study and 69.8% in the WICS-LV Post Market Surveillance Registry had an improvement in their clinical composite score.31,32 There was also a significant reduction in NYHA functional class, QRS duration, improvement in LV ejection fraction (LVEF) and reduction in both LV end-diastolic volume and LVESV.31,32 Overall, 52–55% of patients had a significant reduction in LVESV ≥15%. Additionally, guiding in WiSE-CRT procedures by targeting the electrode to areas of latest activation while avoiding myocardial scar using different imaging modalities has been shown to further improve clinical and echocardiographic outcomes.33,34 In patients who fail to improve following conventional CRT and who undergo WiSE-CRT implantation, 55.6% show improvement in their clinical composite score and 66.7% have a reduction in LVESV ≥15% and/or absolute improvement in LVEF ≥5%.35
Complications
Procedure-related deaths occurred in three of 90 patients (3.3%), with acute complications ≤24 hours after the procedure in 4/90 patients (4.4%), intermediate complications 24 hours–1 month after the procedure in 17 of 90 patients (18.8%), and chronic complications 1–6 months after the procedure in six of 90 patients (6.7%).32 The commonest complications included arterial access complications and cardiac tamponade.
Physiological Pacing and LBBAP
LBBAP and HBP restore physiological activation through the native conduction system, and LBBAP may be more feasible than HBP due to a wider target area.5,36 Although HBP has been shown to lead to narrowing of the QRS duration and cardiac resynchronisation in clinical and simulation studies, implantation can be difficult and the success rates vary from 56% to 95%.37–40 Follow-up can be problematic due to oversensing of atrial signals, undersensing of ventricular signals, lead displacement, and rising capture thresholds with premature battery depletion.36 Indeed, robust long-term data on the outcomes of HBP are currently lacking.
Novel LBBAP was developed to bypass the left bundle branch conduction block by screwing a ventricular lead into the interventricular septum to provide LV resynchronisation.41 Studies have shown it may overcome some of the limitations of HBP.36,42,43 In a prospective study of 341 patients referred for pacing, 30 of whom (8.8%) required CRT, LBBAP was successful in 89% of procedures, and at 1-year follow up the pacing threshold and R waves remained stable.43 Currently, LBBAP is usually delivered using a SelectSecure 3830 pacing lead (Medtronic), and confirmation is dependent on several criteria, which are currently being updated and validated.5,36,44 The predominant complications of LBBAP relate to the risk of septal perforations and lead dislodgements. LBBAP may be affected by intrinsic conduction and programming optimal atrioventricular delays will be important.40
Several studies have shown this to be effective in improving acute haemodynamics and patient outcomes.36,43,45 Several trials have demonstrated the feasibility of LBBAP for delivering CRT.46–49 In a study of 63 patients with non-ischaemic cardiomyopathy, LVEF ≤50%, complete LBBB and who had an indication for CRT or ventricular pacing, left bundle branch pacing was successful in 97% of cases, and this resulted in a significant improvement in LVEF and NYHA functional class at 1 year.48
In a large international multicentre study of 325 patients with LVEF <50% and an indication for CRT or pacing, LBBAP was successful in 85% of patients, and this resulted in significant narrowing of the QRS duration, and improvement in LVEF and NYHA functional class at 6 months.49 Unsuccessful procedures were due to failure to penetrate the septum or inadequate resynchronisation; and the presence of LBBB at baseline was found to be an independent predictor of echocardiographic response.49 Additionally, biventricular pacing was compared with both LBBAP and HBP in a non-randomised observational study of 137 patients with LVEF ≤40%, typical LBBB and referral for CRT.42 It was found that both HBP and left bundle branch pacing resulted in a significant improvement in LVEF and NYHA functional class compared with biventricular pacing at 1-year follow-up.42
LV septal pacing involves pacing the LV endocardial side of the interventricular septum, and this may provide an alternative approach for cardiac resynchronisation. In a study of 27 patients undergoing CRT, temporary LV septal pacing performed via a transaortic approach resulted in a significant reduction in QRS area and standard deviation of activation times, but similar LV dP/dtmax compared with biventricular pacing.50 LV septal pacing may prove to be especially useful in patients who have failed LBBAP, particularly given that the pacing location is relatively large.
Future Directions
LV endocardial pacing with the WiSE-CRT system in prospective registries has demonstrated reliable resynchronisation, improvement of symptoms and reversal of LV remodelling, but the risk of procedural complications requires further evaluation. The ongoing SOLVE-CRT trial is a randomised controlled multicentre trial to assess the safety and efficacy of the WiSE-CRT system, and it will provide important outcome data on the safety and efficacy of leadless LV endocardial pacing.51 In the future, completely leadless pacing and or CRT and defibrillation may be achievable with the incorporation of a Micra transcatheter pacing system (Medtronic), WiSE-CRT system and a subcutaneous ICD (Boston Scientific), but refinements in the technology will be needed before this becomes more widespread.52
LBBAP has the potential to improve outcomes in patients eligible for CRT, and future modifications to the equipment will likely further improve procedural success and patient outcomes. Data on the long-term safety profile and outcomes of heart failure patients who undergo LBBAP for CRT are needed to determine whether this will become a viable treatment intervention. Theoretically, the WiSE-CRT electrode could be targeted to achieve leadless left bundle branch stimulation from the LV endocardium, or HBP from the RV endocardium. However, it is likely that refinements of the technology, including modification of the electrode delivery system, will be required to enable targeted physiological pacing.
Conclusion
Endocardial pacing has many advantages over conventional CRT and has the potential to improve patient outcomes. The WiSE-CRT system allows pacing at a customised location and enables areas of latest activation to be targeted while avoiding myocardial scar. It can lead to clinical improvement, and the ongoing SOLVE-CRT trial will be important in determining its efficacy and safety profile. Physiological pacing with LBBAP has shown promising results in initial trials but its role in CRT requires further investigation. In the future, leadless LBBAP may be achievable but will require technological advances.
Clinical Perspective
- Alternative pacing approaches including left ventricular (LV) endocardial pacing and left bundle branch area pacing (LBBAP) have the potential to provide superior resynchronisation to conventional cardiac resynchronisation therapy (CRT) and to improve response rates.
- Patients undergoing WiSE-CRT implantation should have an assessment of their peripheral arterial vasculature to reduce potential complications. Guiding the electrode to the desired endocardial location can improve patient outcomes.
- Prospective registries have shown that the WiSE-CRT system results in an improved clinical composite score in 70% of patients, and a reduction in LV end-systolic volume ≥15% in 55%.
- LBBAP has the ability to provide physiological pacing and it overcomes many of the problems with His-bundle pacing but its role in CRT requires further investigation.