The prevalence of left bundle branch block (LBBB) in the general population is 0.06–0.1%.1,2 However, LBBB is frequently noted in patients with heart failure (HF), and is associated with higher rates of morbidity and mortality. The prevalence increases from <1% at age 50 years to 6% by 80 years. There is a complex relationship between LBBB and HF, suggested by the prevalence of LBBB in 31% of patients with non-ischaemic cardiomyopathy (NICM), while 21% of patients with LBBB had NICM.3,4 Current guidelines recommend cardiac resynchronisation therapy (CRT) after only 3 months of guideline-directed medical therapy (GDMT) for patients with LBBB and left ventricular ejection fraction (LVEF) <35%. Given the data from CRT trials suggesting patients with LBBB and HF derived maximum benefit compared with those with non-LBBB, and the presence of a baseline LBBB is one of the strongest predictors of superresponse to CRT therapy, it is essential to understand the complex relationship between LBBB and cardiomyopathy.
Definition of Left Bundle Branch Block
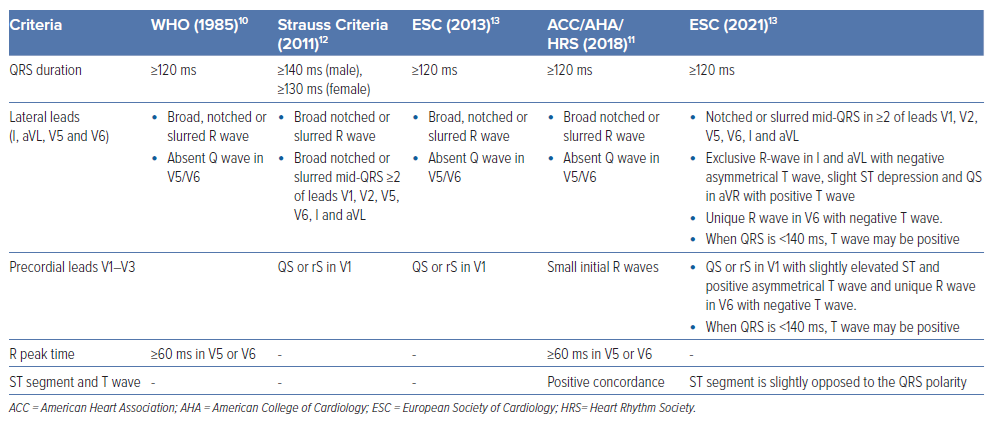
ECG recognition of LBBB was first made in 1914 by Carter et al.5 Although, they mistakenly switched the diagnosis of right bundle branch block and LBBB due to differences in anatomy of humans and canines, which was later corrected by Barker et al. by direct electrical stimulation of the epicardium of the human heart.6 The cut-off of QRS duration >120 ms to differentiate incomplete from complete bundle branch block was proposed in 1941 by Wilson et al. based on canine experiments.7
Grant and Dodge proposed prolongation of QRS duration by >60 ms beyond normal conduction times, along with change in the direction of electrical forces during the initial 30–40 ms on the 12-lead ECG to be defined as LBBB.8 A delay of 40 ms for transeptal conduction time from the right to left side of the septum, 50-ms delay for the electrical impulse to reach the posterolateral wall of the LV and an additional 50 ms for the complete activation of the posterolateral wall resulted in QRS duration of ≥140 ms during LBBB. In the absence of septal infarct or large scar, R-wave amplitude ≥0.1 mV in lead-V1 may exclude the diagnosis of LBBB.9
Current definitions of LBBB are derived based on the WHO consensus criteria, although the majority of these criteria would not identify typical LBBB with right to left septal depolarisation, as these studies included patients with various patterns of conduction delay. Contemporarily, LBBB requires the presence of QRS duration of ≥120 ms, QS or rS in lead-V1, and monophasic R wave with no Q waves in lead I and lead V6.10
The American Heart Association/American College of Cardiology/Heart Rhythm Society guidelines define LBBB as wide QRS morphology with duration ≥120 ms; notched or slurred R wave in leads I, aVL, V5 and V6; absent Q wave in leads I, V5 and V6; R wave peak time >60 ms in V5 and V6 with ST segment; and T wave discordant to the QRS complex.11 Strauss et al. proposed modified criteria to account for the sex difference, and to identify CRT-eligible patients as QRS duration of ≥140 ms in men or 130 ms in women, QS or rS in leads V1 and V2, and mid-QRS notching or slurring in at least two of leads V1, V2, V5, V6, I and aVL.12
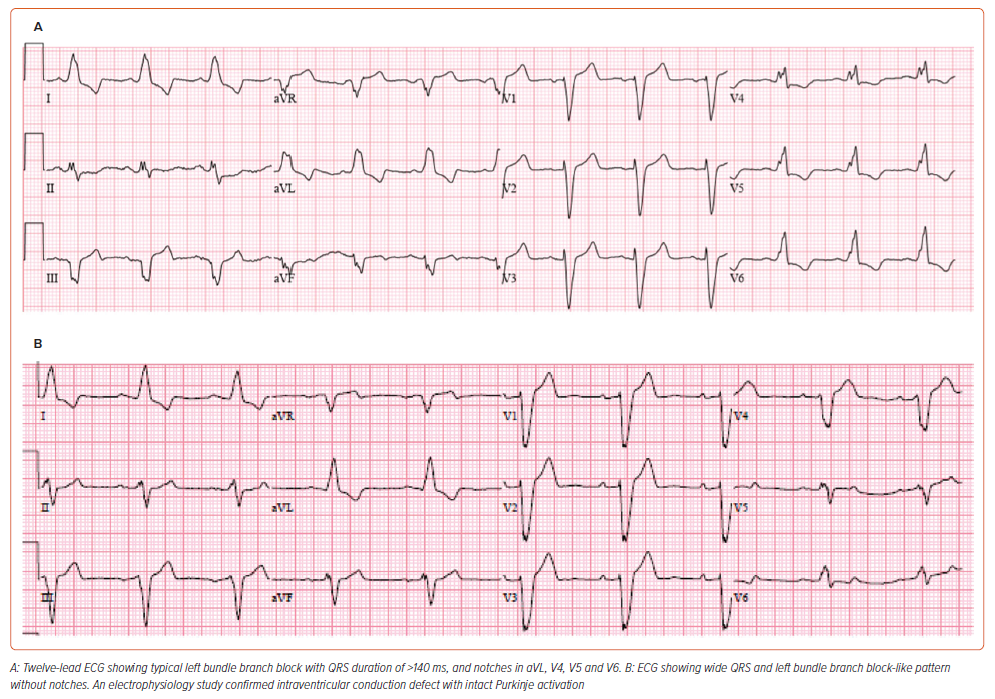
The European Society of Cardiology 2021 criteria define LBBB as wide QRS with duration of ≥120 ms, notching or slurred middle third of QRS in at least two of leads V1, V2, V5, V6, I and aVL with R-wave peak time of >60 ms in V5–V6, discordant QRS and ST segment polarity, QS or rS in V1 with slightly elevated ST and positive asymmetrical T wave, unique R wave in V6 with negative asymmetric T wave, exclusive R wave in I and aVL with a negative asymmetrical T wave, slight ST depression, and usually QS in aVR with positive T wave. These criteria for LBBB are summarised in Table 1. 13 Among these criteria, the ones described by Strauss are most commonly used to describe typical LBBB and predict response to CRT (Figure 1).
Electrophysiology of Left Bundle Branch Block
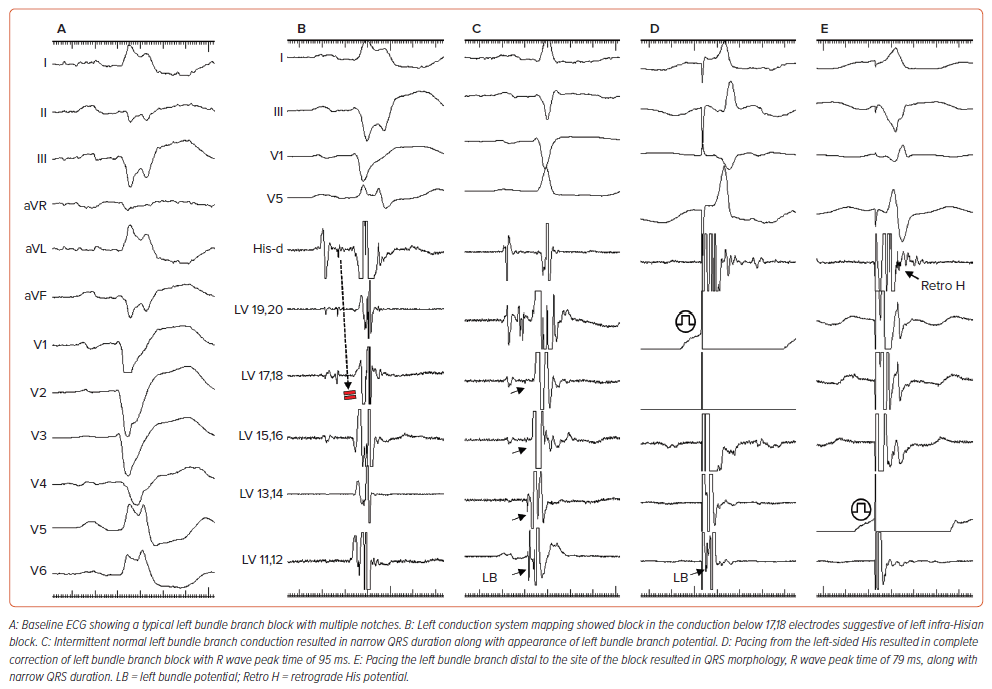
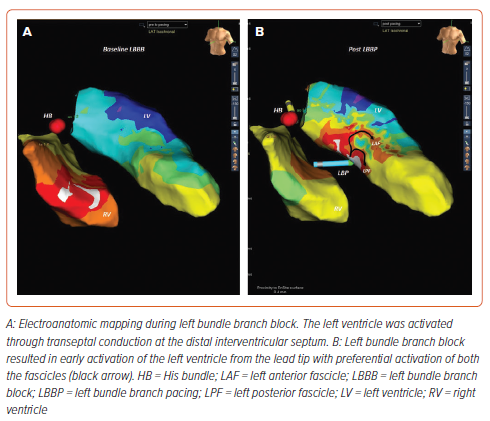
The electrophysiological abnormality resulting in LBBB could be a conduction defect into the left bundle rather than within the left bundle (Figure 2). The fibres inside the His bundle are longitudinally dissociated and, hence, a disease within the His bundle could produce right bundle branch block (RBBB) or LBBB if fibres predestined for RBB or LBB are affected.
Upadhyay et al. performed intracardiac mapping of the left septal conduction to assess the level of conduction block in His-Purkinje system disease.14 Among 72 patients with LBBB, complete conduction block within the proximal left conduction system was observed in 64% (n=46), and intact Purkinje activation in 36% (n=26). Among the patients with block in the conduction system, the site of block was found to be at the level of the His bundle in 72%, and in the proximal left bundle branch in 28%. Additionally, it must be understood that the LBBB pattern was defined based on American College of Cardiology/American Heart Association/Heart Rhythm Society guidelines, and many of the patients undergoing these measurements in this study were undergoing ventricular tachycardia ablation and taking anti-arrhythmic medication (amiodarone).
Understanding the activation of the left His-Purkinje system during complete LBBB is important, as those patients with conduction block into LBBB could be corrected by conduction system pacing, while those with intact Purkinje activation may not be amenable to conduction system pacing.
Left Bundle Branch Block‑induced Cardiomyopathy
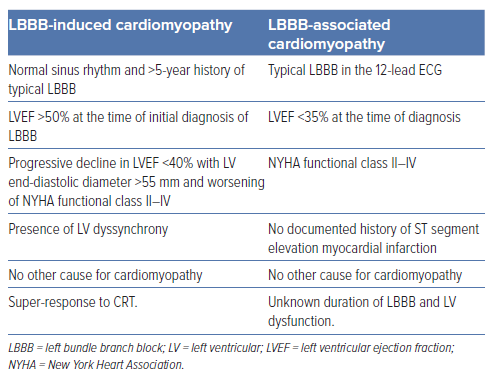
Valliant et al. defined the term LBBB-induced cardiomyopathy (LIC) as having:
- normal sinus rhythm and >5 years history of typical LBBB
- LVEF >50% at the time of initial diagnosis of LBBB
- progressive decline in LVEF <40% with LV end-diastolic diameter >55 mm and worsening of New York Heart Association (NYHA) functional class II to IV
- presence of LV dyssynchrony
- no other cause for cardiomyopathy; and
- super-response to CRT.15
In a retrospective study by Barake et al., LIC was noted in 5.3% (n=134) among 549 patients with LBBB and baseline normal LV function. The mean LVEF dropped from 56 to 31% after an average of 4.6 years.16
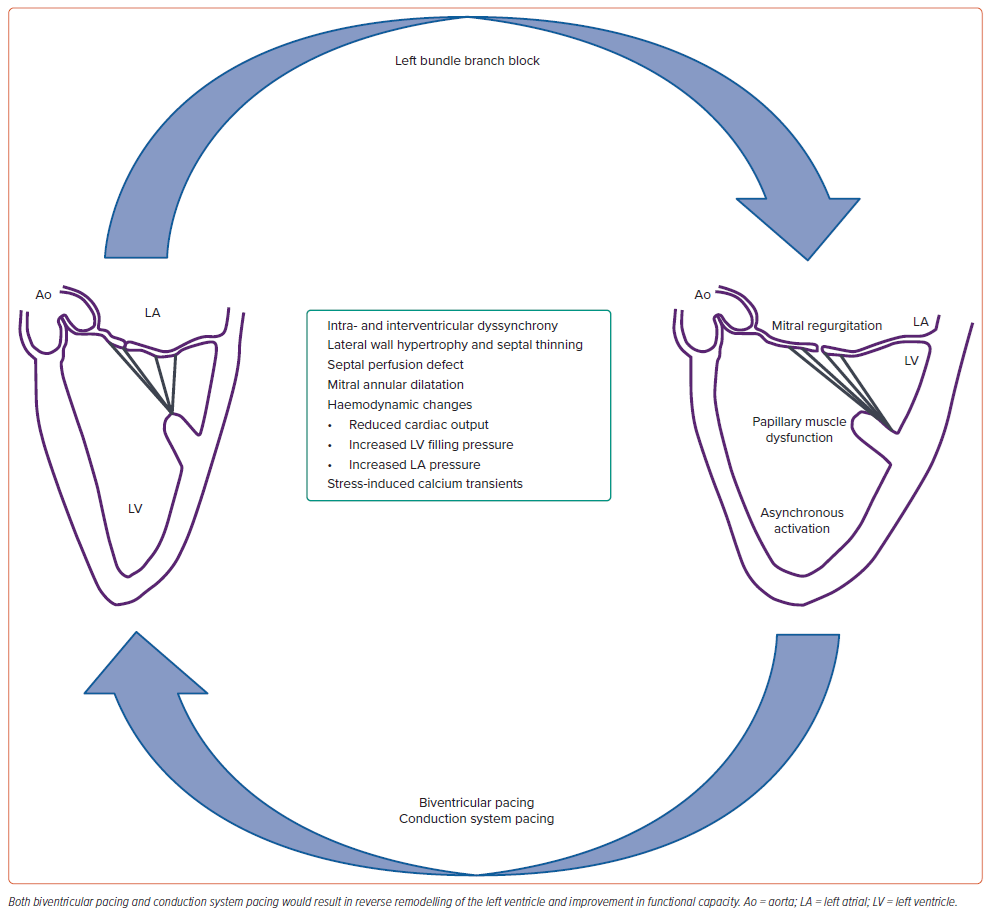
LBBB is characterised by delayed activation of the left ventricular posterolateral wall and significantly prolonged LV activation time resulting in interventricular and intra-ventricular dyssynchrony (Figure 3). Based on echocardiography, three different patterns of interventricular septal movement during systole could be demonstrated.17 Type A and B show abrupt posteriorly directed septal movement during the pre-ejection period, followed by anterior septal movement in type A and posterior septal movement in type B. Type C is characterised by akinetic or dyskinetic septal movement throughout systole.
There is a global discoordination of the contraction/relaxation process designated as intraventricular and interventricular dyssynchrony. Preexcited septal contraction results in displacing the blood towards the fully relaxed lateral wall. Subsequent contraction of the lateral wall displaces the blood back towards the septum, which absorbs the energy from the work performed by the lateral wall. Hence, the loss of septal contribution to LV function, as well as excessive workload on the lateral wall, results in LV lateral wall hypertrophy and thinning of the septum in the majority of the patients with long-standing LBBB.18
Reversible perfusion defects in the interventricular septum in the absence of obstructive coronary artery disease result in intermittent chest pain during LBBB. The mechanism of functional mitral regurgitation includes increased subvalvular traction due to papillary muscle displacement, mitral annular dilatation and slow closure of the mitral valve due to poor LV contraction. LBBB may lead to significant myocardial injury beyond conduction system disease.
In a canine model, ablation of the LBB trunk resulted in fibrotic changes in the left ventricular endocardium and mid-myocardium.19 Purkinje fibres showed fatty degeneration and fibrosis along with downregulation of connexin 43 protein. Apart from the mechanical effects, the dyssynchronous contraction precipitates triggered ventricular arrhythmias by stretch-induced activation of the calcium transients, resulting in generation of after-depolarisation and premature ventricular contractions. All these factors contribute to increased morbidity and mortality in patients with LBBB.
Grines et al. first described the haemodynamic impact of LBBB with the help of apexcardiograms, phonocardiograms, ECGs, 2D and dual M-mode echocardiograms, and radionuclide ventriculograms by including 18 patients with isolated LBBB and 10 healthy control subjects.20 There was a shortening of LV diastole and a resultant increase in the ratio of right to left ventricular diastolic time in LBBB. Radionuclide ventriculograms revealed reduced global ejection fraction due to decreased regional ejection fraction of the septum in LBBB compared with healthy subjects, although apical and lateral regional ejection fractions were similar in both groups.
The effect of LBBB on cardiac function varies between significant reduction in LV function in some patients to minimal effect in others. However, the real challenge is to establish a cause-and-effect relationship between LBBB and LV dysfunction. LBBB may occur during the natural course in HF patients, representing a poor prognostic marker, or it may play a causative role in the development of dilated cardiomyopathy. Patients with LBBB and LV dysfunction often continue to have HF symptoms despite GDMT.21,22 However, CRT implantation is associated with better clinical outcomes compared with patients with LV dysfunction and narrow QRS. This would suggest that in patients with LBBB and dilated cardiomyopathy, the conduction abnormality is the causative factor for the development of cardiomyopathy.
Left Bundle Branch Block-associated Cardiomyopathy
Although considered as one of the reversible cardiomyopathy syndromes, LIC is often difficult to diagnose in clinical practice (Table 2). It is not uncommon for a patient to present with cardiomyopathy and LBBB of unknown duration. Hence, the true prevalence of LIC is often underestimated due to lack of longitudinal ECG or echocardiographic data.
Valliant et al. showed that out of 375 CRT eligible patients, 1.6% (n=6) had LIC.15 However, in another retrospective study, Ponnusamy et al. reported 20% (17/84 patients) prevalence of LIC among patients with LBBB and HF.23 The mean duration between the first diagnosis of LBBB and onset of LV dysfunction was 4.1 ± 3.9 years. Given the evidence that LBBB is the primary aetiology for cardiomyopathy and potentially reversible after CRT, it is essential to identify this subset of patients with LIC as a distinct entity to initiate early device therapy. The term, LBBB-associated NICM (LBNICM), is used to include those patients where the exact duration of LBBB and longitudinal echocardiographic data are not available (Table 2). The criteria for the diagnosis of LB-NICM include:
- typical LBBB in the 12-lead ECG
- LVEF <35% at the time of diagnosis
- NYHA functional class II to IV
- no documented history of ST segment elevation MI
- absence of other identifiable causes of cardiomyopathy; and
- unknown duration of LBBB and LV dysfunction.24
As the presence of typical LBBB in patients with HF translates into superior haemodynamic response to CRT, strict criteria including the duration of LBBB to categorise them as LIC would result in underestimation of the true prevalence. Rather, a broad terminology LB-NICM obviating the duration of LBBB and baseline LVEF at the time of diagnosis of LBBB would help in identifying the potentially reversible form of HF patients.
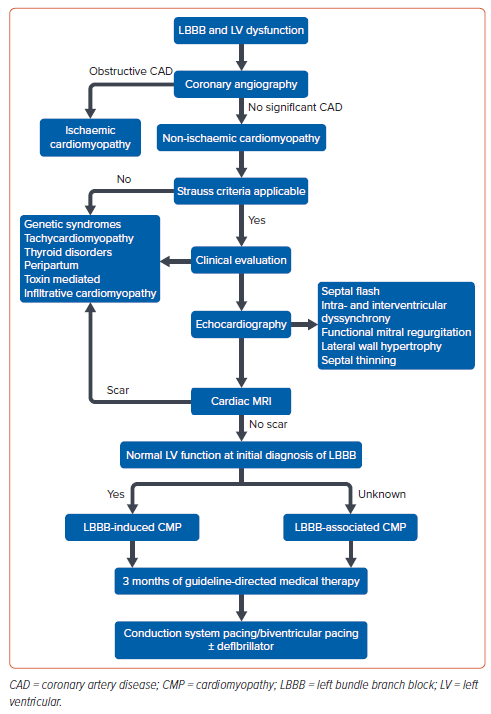
When to Intervene?
LB-NICM is a progressive disease characterised by fixed or variable conduction block into the left bundle and gradual worsening of LV function. In a retrospective study involving 659 patients, LBBB was associated with less improvement in LVEF compared with those with baseline narrow QRS duration even after 3–6 months of GDMT.22 The combined endpoint of hospitalisation due to HF hospitalisation and mortality was higher for LBBB patients. The NEOLITH and NEOLITH II studies demonstrated that LV function did not improve in new-onset LBBB and LV dysfunction with GDMT. Most of these patients would remain CRTeligible candidates.25,26 Delaying CRT beyond 9 months from the time of diagnosis may miss a critical period to halt and reverse progressive myocardial damage. Another prospective study involving NICM patients showed the presence of LBBB as a negative marker of LV reverse remodelling with GDMT alone.27
On the contrary, CRT offers a better outcome in patients with baseline LBBB morphology compared with those with non-LBBB morphology. The presence of LBBB is considered as one of the markers of super-response to CRT. Earlier implantation of CRT was associated with more favourable cardiac remodelling compared with those who received CRT in the later part of the natural history. Even in patients with mid-range LVEF (36–50%), LBBB was associated with significantly higher mortality, worsening of LVEF to <35% and the need for an ICD compared with those without LBBB.28
A prospective study by Zeng et al. showed that early LBB pacing (LBBP), along with GDMT in patients with LBBB and LVEF between 35 and 50%, showed greater improvement in cardiac function and reduced adverse clinical events compared with GDMT alone.29 Given the fixed/progressive nature of the conduction disease and high percentage of super-response to CRT, patients with LB-NICM should be considered for early implantation of CRT to avoid progressive myocardial damage. Future trials are necessary to compare the early implantation of CRT before 3 months of GDMT and in patients with mid-range LVEF (36–50%) compared with current practice.
Role of Defibrillator in Left Bundle Branch Block Non-ischaemic Cardiomyopathy
Prophylactic ICD is a class I indication for patients with HF and reduced LVEF.30,31 The SCD-HeFT trial showed a significant reduction in all-cause mortality with ICD implantation in NICM, the positive effect was confined to those in NYHA functional class II, and none of the patients received concomitant CRT.32 In the DANISH trial, 1,116 patients with NICM were randomised to receive either ICD or usual clinical care along with CRT in 58% of patients in both groups. After a median follow-up period of 67.6 months, ICD implantation was not associated with a significant reduction in mortality compared with the usual clinical care.33 Another prospective study that included 105 patients with low-risk LB-NICM showed LBBP without defibrillator was safe and had the potential to reduce healthcare costs in medium-term follow-up.24
The reasons for the difference in the survival benefits of ICD among ischaemic cardiomyopathy and NICM could be due to the higher incidence of ventricular arrhythmias in the former group and better response to CRT along with reverse remodelling in the latter group. Super-responders had comparable survival to the age–sex-matched general population. LBNICM is especially associated with a high percentage of super-response to CRT, and with the improvement of LV function in these patients, they will no longer qualify for primary prevention ICDs.
Device implantation has its own complications, reported in 6% of patients receiving ICD and 11% of patients receiving CRT defibrillator.34 Even with less-aggressive ICD settings, inappropriate shocks were reported in 6% of patients within 2 years after implantation.35 Normalisation of LV function after CRT, lack of difference in survival among super-responders, procedure-related complications and inappropriate ICD therapies could favour cost-effective CRT alone without defibrillators in low-risk LB-NICM patients.
Risk Stratification of Left Bundle Branch Block Non-ischaemic Cardiomyopathy
As the guidelines still recommend ICD for primary prevention of sudden cardiac death (SCD) in HF patients (ischaemic or non-ischaemic) with ejection fraction <35%, individualised risk stratification based on objective markers would help in identifying LB-NICM patients at risk of SCD with worse clinical outcomes. The ECG markers considered include QRS duration, fragmented QRS, abnormal signal averaged ECG, microvolt T wave alternans and QRS-T angle. The assessment of LVEF by echocardiography is a simple and effective prognostic marker for predicting future adverse cardiac events. Other echocardiographic markers that have also been considered include assessment of LV dyssynchrony, dimensions, atrial size and global longitudinal strain.
Risum et al. studied the role of echocardiographic contraction pattern assessment in predicting the long-term outcome after CRT, and if this was additive to ECG morphology and duration.36 Typical LBBB contraction pattern was defined as demonstration of the following three criteria: early shortening of at least one basal or midventricular segment in the septal wall, and early stretching in at least one basal or midventricular segment in the lateral wall; (2) early septal peak shortening (within first 70% of the ejection phase); and (3) lateral wall peak shortening after aortic valve closure. The absence of a typical LBBB contraction pattern was independently associated with an increased risk of adverse outcome after adjustment for QRS duration and ischaemic heart disease (HR 3.1; 95% CI [1.64–5.88]; p<0.005).
A family history of SCD confers a high risk in cardiomyopathy patients, and a trend towards increased fatal ventricular arrhythmias and HF hospitalisations in gene variant carriers.37 Lamin A/C mutations, seen in 10% of genetic cardiomyopathies, are associated with conduction system disease, fatal arrhythmias, SCD and progression to end-stage heart failure. Filamin-C mutations are noted in 4% of dilated cardiomyopathy cases, and are associated with a high incidence of malignant ventricular arrhythmias and appropriate ICD discharges. RNA-binding motif protein 20, phospholamban, desmoplakin and sodium voltage-gated channel alpha subunit 5 mutations are associated with arrhythmogenic cardiomyopathy and a high incidence of fatal arrhythmias. Hence, a diagnosis of genetic cardiomyopathies with conduction system disease often warrants an ICD implantation as a primary prevention strategy at an early stage.
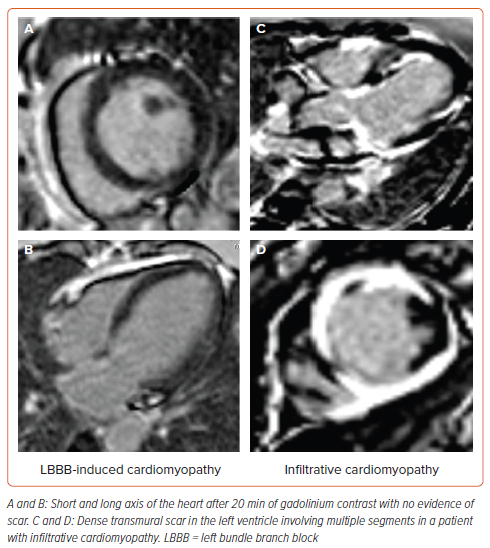
The presence of myocardial scarring is associated with progressive ventricular dilatation, re-entrant ventricular arrhythmias and SCD in NICM patients. Hence, identification and quantification of myocardial scarring has the potential to predict future adverse cardiovascular outcomes (Figure 4). Late gadolinium enhancement cardiac magnetic resonance (LGE-CMR) imaging provides a reproducible assessment of myocardial scarring, and has demonstrated prognostic utility in NICM patients. Incorporating T1 mapping in CMR can further increase the diagnostic yield, as it detects diffuse fibrosis as opposed to LGE, which detects focal fibrosis.
Myocardial scarring has been demonstrated in 38% of NICM patients. In a meta-analysis, Kuruvilla et al. showed that LGE was associated with greater all-cause mortality (OR 3.27; 95% CI [1.94–5.51]; p<0.00001), HF hospitalisation (OR 2.91; 95% CI [1.16–9.27], p=0.02), combined outcome of SCD, aborted SCD or appropriate ICD discharges (OR 5.32; 95% CI [3.45–8.20]; p<0.0001).38
In the MADURAI LBBP study, 120 patients with LB-NICM were categorised into low and high risk based on scar burden (<10 or >10%, respectively) assessment by LGE-CMR.23 Patients with low-scar burden received LBBP without a defibrillator, while those with high-scar burden received LBBP along with a defibrillator. During a mean follow-up of 21 ± 12 months, lowscar burden was associated with better echocardiographic response, normalisation of LV function and lower incidence of death, HF hospitalisation or ventricular tachycardia (VT)/VF. Scar assessment by LGE-CMR would help in predicting the occurrence of ventricular arrhythmias, identifying the target site for coronary sinus lead deployment and response to CRT. The presence of septal scarring is considered as one of the limiting factors for successful LBBP. A retrospective study demonstrated transmural scaring in the LBBP zone predicted procedural failure with 100% sensitivity and specificity.39
A majority of studies included LVEF as a prognostic marker for predicting future cardiovascular outcomes in HF patients. In patients with NICM, combining LVEF assessment and LGE-CMR for risk stratification would help in assessing the long-term prognosis, response to CRT and the need for defibrillator therapy.
Long-term Outcomes of CRT
CRT by biventricular pacing (BVP) has been the standard treatment for patients with cardiomyopathy and wide QRS duration to improve the HF symptoms, quality of life and LV function. Although several multicentre studies have demonstrated the presence of baseline LBBB as a predictor of super-response to CRT, the true prevalence of typical LBBB among the study cohorts would be difficult to determine due to wide variability in defining LBBB.
Biventricular Pacing
Biventricular pacing reversed the electrical and mechanical abnormalities found in the left ventricle in the animal model of isolated LBBB, reinstating the concept that LBBB might be considered as a reversible cause of NICM.40 The MADIT CRT trial was designed to assess the efficacy of biventricular pacing by randomising 1,820 patients with ejection fraction <30%, QRS duration of >130 ms and NYHA functional class I or II symptoms.41 Baseline LBBB was noted in 70% of the study population, and NICM in 45% of the study population. The presence of LBBB predicted super-response, with an OR of 2.05 (95% CI [1.24–3.40]; p=– 0.006), along with a reduction in HF and all-cause mortality (HR 0.57; 95% CI [0.34–0.95]; p=−0.029).42 In patients with LBBB, assessment of mechanical dyssynchrony by echocardiography remains controversial, as it failed to improve the selection of patients or to predict the response after BVP.43
Blanc et al. first described LBBB as a reversible form of dyssynchronyinduced cardiomyopathy. Complete normalisation of LV function (LVEF >50%) at 1 year was noted in 17% (n=5) of patients with NICM with LBBB after BVP.44 The authors suggested long-standing LBBB as a newly identified reversible cause of cardiomyopathy. Another prospective study involving 51 NICM with LBBB patients showed complete functional recovery and normalisation of LV function in 21.5% (n=11) of patients.45 Valliant et al. defined LBBB-induced cardiomyopathy with a low prevalence (1.6%) among 375 CRT eligible patients. HF developed over a mean period of 11.6 years.15 After CRT, mechanical dyssynchrony resolved in all patients, with improvement in LVEF from 31 ± 12% to 56 ± 8%.
Wang et al. showed that in patients with LB-NICM, early initiation of CRT therapy was associated with favourable cardiac remodelling.26 Among 105 patients, LVEF improvement to >35% was more likely in those implanted <9 months compared with >9 months (OR 3.53; 95% CI [1.32– 9.46]; p=0.01).
Based on current guidelines, the decision to implant CRT should be strongly considered immediately after 3 months of GDMT, as the majority of these patients would be still symptomatic, and CRT has the potential to reverse remodel the LV with early implantation. Future studies are warranted to assess the CRT implementation within 3 months of diagnosis in patients with LB-NICM, a dilated left ventricle and markedly reduced LVEF, as these patients are not expected to have an improved outcome with GDMT alone.
Conduction System Pacing
Nearly one-third of patients receiving BVP do not respond favourably, despite improvements in delivery catheters and dedicated leads. Conduction system pacing has been suggested as an alternative modality to provide CRT, as it provides synchronised activation of the ventricles correcting the electromechanical delay by pacing distal to the site of conduction block.
Deshmukh et al. first demonstrated the human feasibility of His bundle pacing in patients with chronic AF and HF.46 Fibres inside the His bundle are thought to be longitudinally dissociated and predestined for the right or left bundle branches. Permanent His bundle pacing (HBP) could correct typical LBBB in nearly 72% of patients who are likely to have complete conduction block within the His bundle.
A retrospective study by Singh et al. showed the efficacy of HBP in patients with LIC (n=7).47 The mean LVEF increased from 25 to 50% (p=0.0001), with all patients achieving echocardiographic hyperresponse between 3 and 13 months.
In an observational study, Huang et al. assessed the efficacy of HBP to correct typical LBBB in 74 patients with HF and its long-term clinical outcome.48 LBBB correction could be acutely achieved in 97.3% (n=72) of patients, while 75.7% (n=56) of patients received permanent HBP. At 3-year follow-up, LVEF increased from 32.4 ± 8.9% to 55.9 ± 10.7% (p<0.001). Normalisation of LV function (ejection fraction >50%) was observed in 88.9% of patients who had baseline LVEF <40%. However, the acute threshold for LBBB correction was 2.13 ± 1.19 V/0.5 ms, although it remained stable (2.29 ± 0.92 V/0.5 ms) at 3-year follow-up.
To overcome the limitations of HBP, Huang et al. suggested direct capture of LBB fibres on the LV subendocardium by deep septal lead placement.49 As the lead is deployed in the distal conduction system, LBBP has the potential to correct the majority of conduction system disease (Figure 5). LBBP provides low and stable thresholds, along with excellent lead stability.
A retrospective study by Ponnusamy et al. showed 17 (20%) out of 84 LBBB patients had possible LIC.23 LBBP was performed in 13 patients. The mean LVEF dropped from 53.6 ± 2.4% at baseline at the time of LBBB diagnosis to 30.5 ± 6.7% before LBBP, which further increased to 57.4 ± 4.7% after LBBP. All patients had normalisation of LV function between 3 and 6 months after LBBP. Huang et al. demonstrated a 97% success rate (61/63 patients) for LBBP in patients with typical LBBB, NICM and LVEF <50%.50 Normalisation of LV function was achieved in 75% of the patients at the end of 1 year.
In a large non-randomised multicentre study by Vijayaraman et al., baseline LBBB was noted in 1,073 out of 1,778 patients with LVEF ≤35% having class I or II indications for CRT.51 Among patients with LBBB, echocardiographic hyperresponse was observed in a significantly higher percentage of patients with LBBP compared with BVP (42.1 versus 28.5%; HR 1.771; 95% CI [1.305–2.402]; p<0.001). Similarly, there was a greater reduction in clinical outcomes of death or HF hospitalisation in the LBBP group compared with the BVP group.
Diaz et al. compared the outcomes between LBBP and BVP as an initial implant strategy for patients with LBBB-associated cardiomyopathy (LVEF ≤35%) and those with LVEF <40% requiring >40% right ventricle pacing. LBBP was associated with shorter procedural duration, narrow QRS duration and higher postprocedural LVEF compared with BVP. The primary efficacy outcome was defined as the composite of HF hospitalisation, and all-cause mortality occurred in 24.2% of the LBBP group compared with 42.4% in the BVP group (HR 0.621; 95% CI [0.45–0.93]; p=0.021).
In the MADURAI LBBP study, 120 patients of LB-NICM were prospectively enrolled and risk stratified based on CMR to receive LBBP alone or LBBP with a defibrillator.15 LB-NICM patients with a low-scar burden had a better clinical outcome with a LBBP optimised dual chamber pacemaker, with 80% of patients having normalisation of LVEF at the end of 1-year followup. The primary composite endpoints of death, HF hospitalisation or VT/ VF were observed in only 3.8% of patients with low-scar burden compared with 33.3% of patients with high-scar burden. There were no episodes of SCD in patients who received LBBP alone. Hence, adding CMR for risk stratifying LB-NICM patients would help in providing cost-effective CRT with superior haemodynamic response.
Sex-specific differences in response to CRT by LBBP were similar to BVP, and women had a greater reduction of death or HF hospitalisation among those with NICM and LBBB.52 LBBP has been shown to be associated with a low incidence of sustained VT/VF and new onset AF compared with BVP.51
In a retrospective study that involved 1,414 propensity score-matched CRT eligible patients, Herweg et al. showed that the occurrence of VT/VF was significantly lower in LBBP compared with BVP (4.2 versus 9.3%; HR 0.46 95% CI [0.29–0.74]; p<0.001).53 Among patients with no previous history of AF, the occurrence of new-onset AF lasting for >30 s was significantly lower with LBBP than with BVP (2.8 versus 6.6%; HR 0.34; 95% CI [0.16– 0.73]; p=0.008). Baseline LBBB morphology was noted in 57.7% of patients in the LBBP arm and 59.3% in the BVP arm. Physiological resynchronisation by LBBP resulting in decreased left atrial pressure, reverse atrial remodelling and near normal activation of the left ventricle may lower the risk of atrial and ventricular arrhythmias compared with BVP.
Although there are no large head-to-head comparisons of BVP with LBBP for LB-NICM, several non-randomised studies have shown that conduction system pacing resulted in a higher percentage of super-responders compared with BVP among patients with LIC and LB-NICM. Direct stimulation of the conduction system, superior electromechanical resynchronisation (Figure 5) and a reversible form of conduction system disease-related cardiomyopathy as a substrate are the possible reasons for excellent clinical outcomes after LBBP.
Areas of Uncertainty
Not all patients with LBBB will develop LV dysfunction, and the prevalence varies widely. Similar to pacing-induced cardiomyopathy, the reason behind worsening of LV function only in a few patients is largely unknown. LV function at the time of onset of LBBB may not be available for all patients and, hence, strict criteria for the diagnosis of LIC might miss the potential patient cohort for whom a superior haemodynamic response to CRT is expected. Rather, LB-NICM would be the clinically appropriate terminology to select patients and to offer better resynchronisation.
A non-randomised comparison showed LBBP to be superior in patients with NICM with LBBB compared with BVP. However, further randomised trials are required to confirm the superiority before considering LBBP as a first-line strategy for these patients. The conduction disease in LB-NICM is fixed, progressive and amenable to complete correction by conduction system pacing. Hence, the role of LBBP immediately after diagnosis rather than waiting for 3 months of GDMT needs to be tested in future trials. A systematic approach would help in risk categorising patients, and identifying the subset with a reversible form of conduction system disease-related cardiomyopathy (Figure 6).
Conclusion
LBBP is a common finding in patients with symptomatic HF, and is considered as a marker of super-response after CRT. LB-NICM represents a potentially reversible form of cardiomyopathy, with nearly 75% of patients having LV reverse remodelling after LBBP. Future multicentre, prospective studies would help in validating the diagnostic criteria, planning the time of implantation and comparing BVP versus LBBP.
Clinical Perspective
- The presence of left bundle branch block in patients with heart failure is one of the strongest predictors of response to cardiac resynchronisation therapy.
- The Strauss criteria are the most commonly used to describe an electrocardiographic pattern of typical left bundle branch block and predict the response to cardiac resynchronisation therapy.
- In left bundle branch block-associated cardiomyopathy, the conduction abnormality is the causative factor and, hence, considered as a reversible form of cardiomyopathy.
- Left bundle branch pacing provides superior electrical and mechanical resynchronisation, with nearly three-quarters of patients having reverse remodelling of the left ventricle.