AF is one of the most prevalent types of arrhythmia encountered in the adult population in daily clinical practice, and it has long been associated with morbidity (e.g. stroke, heart failure) or even an increased risk of mortality.1,2 Several trials have indicated that catheter ablation is superior to anti-arrhythmic medications (AADs) for the treatment of AF.3–5 Despite significant attempts directed at improving procedures and technologies to achieve better pulmonary vein isolation (PVI), arrhythmia recurrence remains a considerable issue, most frequently brought about by a failure to establish durable lesions around the pulmonary veins (PVs).6,7
With state-of-the art advances that have seen integration of contact force-guided sensors into focal radiofrequency ablation (RFA) catheters and cryogenic balloon catheters capable of producing PVI with a single ablation lesion, the duration of procedures has lengthened substantially, placing limits on the number of ablations that may be accomplished on any given day. This has contributed to much longer times from diagnosis to ablation, further increasing morbidity and AF recurrence.8,9 Furthermore, considering thermal energy does not target the cardiac muscle directly, collateral tissue damage may occur, possibly leading to serious adverse effects, such as oesophageal injury, PV stenosis and the risk of thromboembolic events through tissue coagulation.10 As a result, constant technological advancements are sought to improve the effectiveness and safety profile of the procedures.
Irreversible electroporation of cardiac myocytes by pulsed field ablation (PFA) has emerged as an entirely novel non-thermal energy source that generates sufficiently profound lesions with strong lesion durability, and no discernible extracardiac damage due to its increased selectivity towards cardiac myocytes. Because cell membranes are affected by electromagnetic fields, assuming the applied force is strong enough to exceed the transmembrane voltage, electrical conductivity and membrane permeability are altered by the formation of aqueous pores. This promotes the transmembrane passage of normally impermeable substances, altering the cell’s integrity.10,11 Nevertheless, because PFA is a relatively new technology, there are limited data from real-world experience, which raises safety concerns. Although a meta-analysis conducted in 2023 by Aldaas et al. demonstrated that there is no statistically significant difference in the rate of recurrent atrial arrhythmias between PFA and thermal ablation, that meta-analysis merely examined single-arm trials, which reduced the generalisability of the findings to groups not included in the study and comparisons to other studies because reported rates may be attributable to factors other than the experimental regimen.12 Thus, the aim of the present meta-analysis was to summarise the most recent evidence and compare the efficacy and safety of PFA PVI to that of thermal ablation (RFA and cryoablation) in patients with AF. Our aim was to undertake a more elaborate comparison analysis, with numerous additional subgroup studies, a meta-regression analysis and a detailed discussion to provide novel but credible insights into this issue.
Methods
Protocol and Registration
This systematic review was conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions and is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.13,14 The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO; ID: CRD42023484508).
Literature Search Strategy
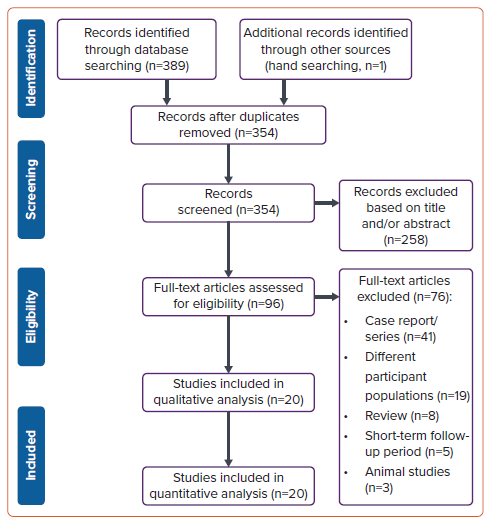
The PubMed, Europe PMC, and ScienceDirect databases up to November 2023 were searched. The search terms were as follows: ((pulsed field ablation) OR (irreversible electroporation)) AND (atrial fibrillation) AND (catheter ablation). When required, the reference lists of the included research and relevant review papers were scrutinised for additional references. We tailored the search keywords to the particular requirements of each database. Our search followed PRISMA principles, with the search and screening procedures shown in the flowchart in Figure 1.
Study Selection
We included randomised controlled trials, observational studies (both prospective and retrospective) reporting detailed periprocedural characteristics and outcomes in PFA catheter ablation and studies comparing the efficacy, safety and outcomes (AF recurrence, PVI durability at the most recent available follow-up and successful rates of PVI at the end of the procedure) of PFA with those of thermal catheter ablation (either RFA or cryoablation). We omitted studies that failed to provide sufficient data for the aforementioned categories. Animal studies, review papers, editorials, comments, letters to editors, case reports/series, meta-analyses and conference abstracts were also excluded from our meta-analysis.
Intervention Versus Control Groups
The intervention group comprised AF patients undergoing PVI using the PFA technique, which causes lesions in cardiac tissue non-thermally and in milliseconds via the irreversible electroporation process.15 The control group comprised patients with AF undergoing PVI with conventional ablation (either RFA or cryoablation) using any ablation technique (including contact force-guided sensor and ablation-index guided process). The PFA included studies used the FARAWAVE ablation catheter (Farapulse Inc.) to conduct the ablation process.
Our research protocol allowed for investigations encompassing both PVI alone and PVI with additional ablation beyond the PVI, such as left atrial posterior wall (LAPW) isolation, a substrate modification approach using complex fractionated atrial electrogram ablation and linear ablation, and cavotricuspid isthmus (CTI) ablation; in these cases, additional analyses were performed in connection with these procedures.
Outcomes of Interest
The primary outcome of this study was AF recurrence. AF recurrence was defined as AF and atrial tachycardia events lasting more than 30 s following ablation at least 3 months after the index procedure (blanking period). For arrhythmia detection, follow-up data were collected at outpatient clinic visits at 3, 6 and 12 months after the PVI. Before each appointment, a 24- to 72-h Holter ECG was done to monitor for any recurrence of atrial arrhythmias. Secondary study outcomes were PVI durability, total procedure time (in minutes), fluoroscopy time (in minutes) and complications related to the procedure. PVI durability was measured as the fraction of PVs that remained durably isolated upon invasive reassessment. Complications related to the procedure included pericardial effusion/tamponade, vascular access complications arising from catheter ablation, phrenic nerve palsy, thromboembolic events, coronary spasm and oesophageal injury/fistula.
Data Extraction and Risk of Bias Assessment
Data were extracted independently by two authors (MI and WK) using a form. The information collected included the baseline characteristics of individuals included the studies (e.g. age, sex), study design, the country in which the study was conducted, BMI, the study population, AF types, hypertension, diabetes, stroke/transient ischemic attack, coronary artery disease, heart failure, the use of AADs, left atrial size, left ventricular ejection fraction, CHA2DS2-VASc score, catheter used, thermal ablation methods, additional ablation characteristics (both PFA and thermal ablation), additional ablation beyond PVI and follow-up modality and time points.
The Newcastle–Ottawa Scale was used to independently assess the possibility of bias in each study.16 A study with a total score of ≥7 was deemed bias-free. Studies with a total score of ≤6 were considered to be biased, and thus were excluded from the meta-analysis. Author discussions were used to settle disagreements regarding quality rating.16
Statistical Analysis
In this meta-analysis we used Stata 17 and Review Manager 5.4 to calculate the overall effect size. The Mantel–Haenszel method and generic inverse variance approach were used for dichotomous and continuous data, respectively. For single-arm studies, we used meta-analysis of proportion for every event per total. RRs were used to measure binary comparisons, whereas the mean difference was used to estimate continuous variable comparison as an effect size. I2 was used to measure the heterogeneity of pooled estimates, with I2>50% or p<0.10 denoting statistically significant heterogeneity. A random-effects model was used for the analyses, regardless of heterogeneity, to calculate the pooled effect size. A restricted maximum likelihood method was used to identify any confounders based on the baseline and clinical characteristics of individuals throughout the incidence and comparison of AF recurrences between PFA and thermal ablation groups. Only variables that were reported by a minimum of 10 studies were analysed in the aforementioned analysis. Subgroup analyses were also performed for additional ablation beyond PVI and AF classification. The Egger test was used to quantify publication bias. All statistical analyses were two-sided, with statistical significance set at p<0.05.
Results
Study Selection and Characteristics of the Included Studies
Figure 1 shows the results of the literature search. After removing duplicates from the 389 articles originally identified in the search, the titles and abstracts of the remaining 354 articles were reviewed, and a further 258 articles were excluded. The full text for all remaining 96 articles was obtained and the studies further screened for eligibility. Seventy-six studies were deemed ineligible, with 20 studies finally included in the qualitative and quantitative analyses.15,17–35 Among these 20 studies, there was one randomised controlled trial, four prospective and four observational studies and 11 single-arm prospective and retrospective studies. The mean age of the participants across all studies was 63.4 years and 65.4% were male (Supplementary Table 1).
All studies from the intervention group (PFA) used the FARAWAVE ablation catheter and patients were treated with a set of microsecond-scale biphasic pulses of 1,800–2,000 V. PVI was performed with four applications in a basket configuration and four applications in a flower configuration per PV. Nine studies reported only paroxysmal AF and one study reported only persistent AF, whereas the other 10 studies reported both paroxysmal and persistent AF. Five studies went beyond PVI, specifically blocking the LAPW and CTI. Three studies compared PFA and cryoablation, two studies compared PFA and RFA and four studies compared PFA with both RFA and cryoablation as the control group. The duration of follow-up ranged from 3 to 12 months after the procedure, with Holter monitoring performed after a 3-month blanking period (Supplementary Table 2).
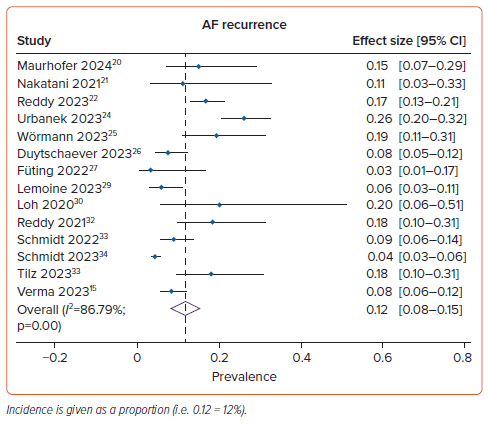
AF Recurrence
The mean (±SD) duration of follow-up was 8.6±4.1 months. AF recurrence was seen in 12% (95% CI [8–15%]; I2=86.79%; p<0.001) of the PFA group (Figure 2) and in 25% (95% CI [19–31%]; I2: 71.70%; p<0.001) of the control group. However, the pooled results of six comparative studies suggest that there was no significant difference in the risk of AF recurrence between the PFA and control groups (RR 0.90; 95% CI [0.70–1.17]; p=0.44; I2=22%; Figure 3). The incidence of AF recurrence was not markedly altered by age, male sex, BMI, paroxysmal AF, hypertension, diabetes, stroke/transient ischemic attack, coronary artery disease, heart failure, use of AADs, left atrium diameter, left ventricular ejection fraction, CHA2DS2-VASc score or additional ablation beyond the PVI (p>0.05). Subgroup analysis revealed that the rate of AF recurrence in groups with and without additional ablation beyond the PVI was 10% (95% CI [4–17%]; I2=91.82%; p<0.001) and 13% (95% CI [8–17%]; I2=77.74%; p<0.001), respectively (Supplementary Figure 1A). Further subgroup analysis indicated that the rate of AF recurrence in the predominantly paroxysmal AF and persistent AF groups was 14% (95% CI [8–20%]; I2=84.39%; p<0.001) and 9% (95% CI [5–12%]; I2=74.09%; p<0.001), respectively (Supplementary Figure 1B). In the persistent AF population specifically, subgroup analysis indicated that the rate of AF recurrence in groups with and without additional ablation beyond the PVI was 4% (95% CI [3–6%]) and 11% (95% CI [7–15%]; I2=44.84%; p=0.12), respectively (Supplementary Figure 1C). Additional subgroup analysis based on follow-up duration showed that the rate of AF recurrence was 13% (95% CI [8–17%]; I2=91.13%; p<0.001) for a follow-up duration of 12 months and 9% (95% CI [4–14%]; I2=38.68%; p=0.16) for a follow-up duration of <12 months (Supplementary Figure 1D). No significant heterogeneity between articles was found in the comparative analysis (Figure 3).
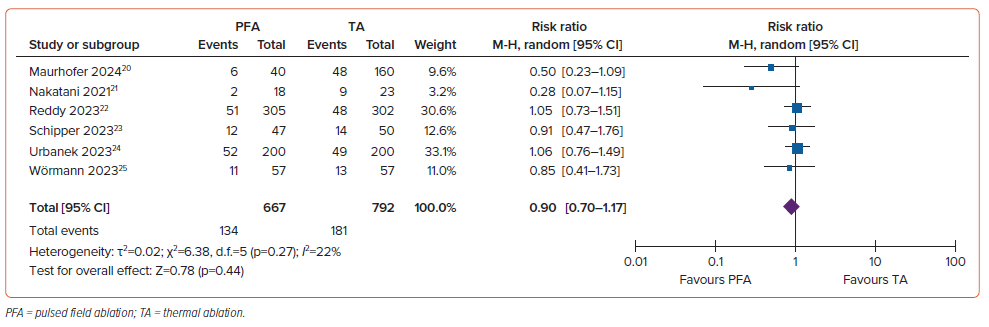
Pulmonary Vein Isolation Durability
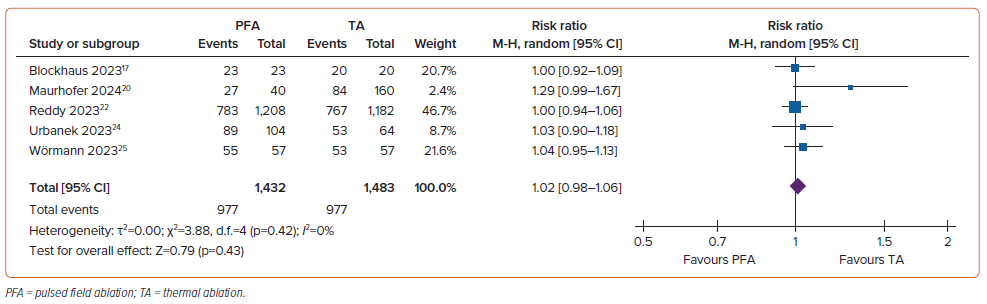
The rate of PVI durability was 83% (95% CI [65–99%]; I2=98.87%; p<0.001) in the PFA group and 79% (95% CI [60–98%]; I2=98.80%; p<0.001) in the control group. There was no significant difference in PVI durability between the two groups (RR 1.02; 95% CI [0.98–1.06]; p=0.43; I2=0%; Figure 4). There was no substantial heterogeneity across studies.
Periprocedural Time
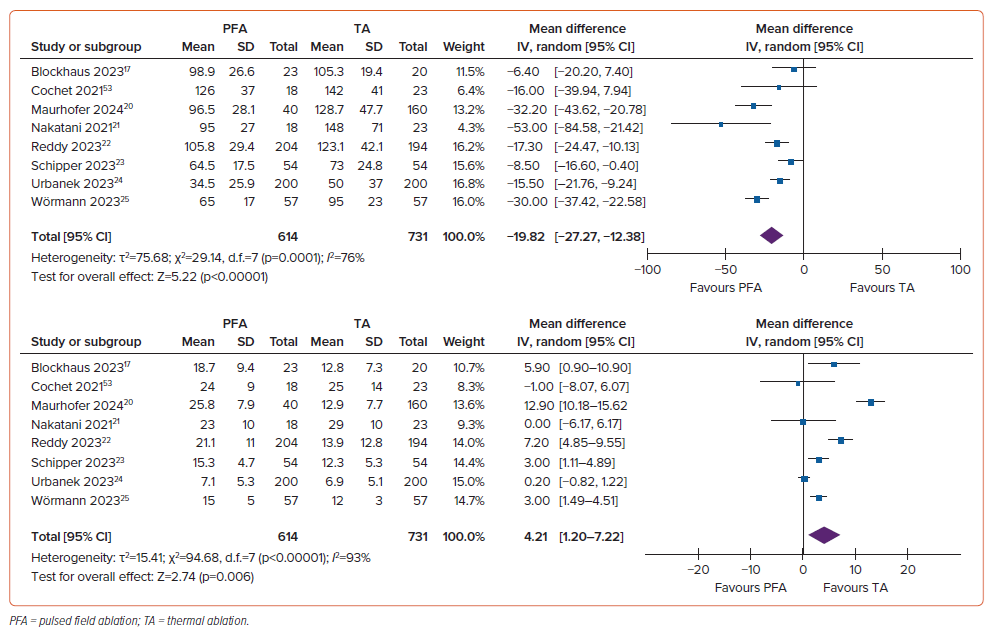
Procedure duration was considerably shorter in the PFA group, with a mean difference of –19.82 minutes (95% CI [–27.27, –12.38 minutes]; p<0.001; I2=76%). Conversely, the duration of fluoroscopy was substantially longer in the PFA group, with a mean difference of 4.21 minutes (95% CI [1.20–7.22 minutes]; p=0.006; I2=93%; Figure 5).
Complications
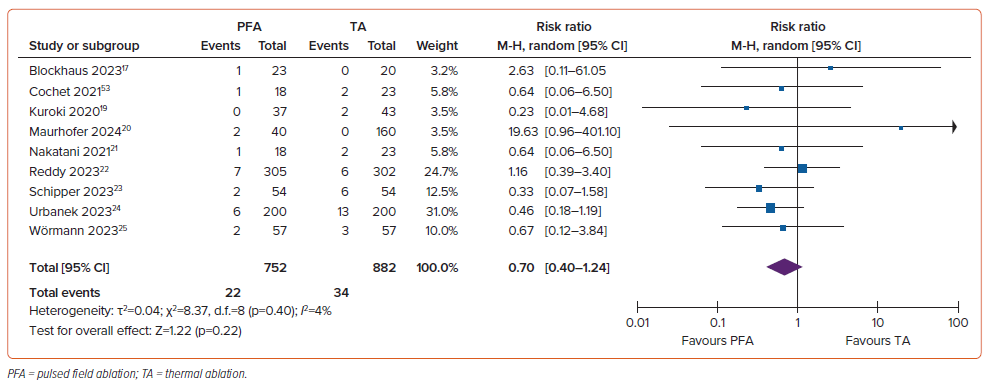
The rate of complications was comparable in the two groups (RR 0.70; 95% CI [0.40–1.24]; p=0.22; I2=4%; Figure 6). Most complications were linked to catheter implantation, such as effusion and tamponade, which were documented in 32 of 2,322 (1.4%) patients in total. Further complications associated with vascular access, including bleeding and groin hematoma, were found among 30 of 2,356 (1.3%) individuals. Other complications included cerebrovascular accident in 21 of 2,140 (0.9%) individuals, temporary phrenic nerve palsy in 5 of 2,047 (0.2%) individuals, reversible coronary spasms in 3 of 1,298 (0.2%) individuals and unspecified adverse events in two individuals.
Publication Bias
The Egger test was used to evaluate publication bias and showed that a small study effect was not detected for the efficacy of PFA compared with thermal ablation in terms of AF recurrence, PVI durability, periprocedural time and complications (p>0.05).
Discussion
The most noteworthy finding of this study is that total procedure time was shorter but fluoroscopy time was longer for patients who underwent PFA compared with those who underwent thermal ablation for PVI of AF. Moreover, AF recurrence, PVI durability and periprocedural complications were comparable between the two procedures. To the best of our knowledge, this meta-analysis is the most comprehensive in comparing PFA and thermal ablation in terms of efficacy, safety and outcomes for electrical isolation of PVs, as discussed below.
Although these may be anticipated given the same extent of AF recurrence and PVI durability between the PFA and thermal ablation groups, the processes behind these are presumably more complicated. Even though it appears that PVI durability rates were lower in the thermal ablation than PFA group, it is speculated that PFA may result in more transmural lesions with a lower incidence of PV reconnection, but with insufficient ablation of the adjacent ganglionated plexi, which has been implicated in the development of AF via autonomic nervous system activation, eliciting a net recurrence of atrial arrhythmias comparable to that seen after thermal ablation.21,36,37 However, this was disproven by Schipper et al., who found no discernible differences in heart rate alterations between PFA and thermal ablation, which was further corroborated by a recent prospective randomised trial that found no improvement in outcomes following ablation of ganglionated plexi.23,38,39
AF recurrence remains a challenge, thus it is vital to understand the various pathways by which AF can reoccur following catheter ablation. Late recurrence during the first 9 months following the blanking period occurs in 25–40% of patients, with the prevalence of late recurrence varying according to AF characteristics (paroxysmal versus persistent).41,42 In the present study, the results of subgroup analysis showed that the rate and effectiveness of PFA in decreasing AF recurrence remained comparable among individuals who did not have further ablation beyond PVI (Supplementary Figure 1A). However, in the persistent AF population, those who underwent additional ablation beyond PVI generally had a slightly lower incidence of AF recurrence than those who did not, with the difference failing to reach statistical significance due to the limited number of studies (Supplementary Figure 1C). There is insufficient data to justify the adoption of additional ablation procedures other than PVI, such as LAPW and preventive CTI ablation, because they have not shown substantial therapeutic benefits.43,44 This meta-analysis reinforces the findings of previous investigations, suggesting additional ablation may not be required. However, more research comparing PFA ablation with and without additional ablation beyond PVI is required to corroborate this conclusion. This highlights the evidence gap from PFA being categorised as a novel approach in the domain of catheter ablation for PVI, leaving some unanswered questions, namely ‘how much is enough and how much is too much?’.
From a technological point of view, when cell membranes are subjected to electromagnetic fields, provided the applied force is high enough to exceed the transmembrane voltage, electrical conductivity and membrane permeability are changed by the formation of aqueous pores. This encourages the transmembrane passage of typically impermeable substances, compromising the cell’s integrity and resulting in cell death.11 Furthermore, the importance of an optimum pulsed electric field protocol became apparent in this study because participants who were treated with an optimised biphasic waveform had more lasting PVI than patients who were treated with an initial monophasic waveform. In addition to an increase in tissue temperature due to energy dissipation, another infrequently considered secondary impact of the application of monophasic pulses is the generation of gaseous microbubbles due to electrolysis, further emphasising the need to establish appropriate electroporation protocols.44,45 Another finding with PFA is the loss of acute late gadolinium enhancement in the chronic period.21 Structural breakdown of the matrix during thermal ablation may expose fibroblasts to mechanical stress, resulting in persistent fibrosis. However, the intact extracellular matrix frame after PFA may shield fibroblasts from mechanical stress, preserving tissue compliance.21,46
As anticipated, the total procedure time was notably reduced in patients who underwent PFA compared with those who underwent thermal ablation. However, fluoroscopy time was longer in the PFA than thermal ablation group, which may be explained by a long learning curve for PFA. Operator inexperience and the widespread use of non-fluoroscopic, 3D mapping devices with thermal ablation seem to be the most likely explanations. The fluoroscopy time should decrease as operators become more experienced with PFA and as mapping systems are integrated with PFA in the future.
In the case of single-shot approaches comparable to cryoablation, PV anatomy must be considered, as well as whether CT or other imaging modalities are essential for evaluating PV architecture or size.47 However, at this point, no studies have reported anatomical limitations for PFA, possibly due to its flexibility and durability in creating any desired lesion set in either a flower or basket configuration.48 Only one study performed a baseline chest CT or MRI scan to facilitate future characterisation of any probable PV stenosis occurrences; however, patients were not excluded based on PV anatomy or size.15
In terms of safety, it is worth noting that PFA has a relatively low frequency of adverse events, and the rate of complications in the PFA and thermal ablation groups was comparable. Several complications appear to be worth investigating and seem to be preventable. First, tamponade has been linked to the use of a straight, extremely rigid guidewire, which was initially used to guide catheter location but resulted in perforations and, eventually, tamponade. For this reason, the use of a J-tip guide wire was proposed, and, interestingly, no more tamponade has been observed.23–25 Second, cerebrovascular accidents occurred in a substantial number of patients who underwent PFA ablation. Because there is no preprocedural cerebral imaging to serve as a comparison, there is concern about the number of procedure-related silent cerebral events in patients with AF, who are already predisposed to such events. Most of these episodes are asymptomatic and resolve on their own; however, appropriate therapy for cerebrovascular accidents may decrease the number of neurological adverse events.17,33,34,49 Third, 0.2% of patients experienced transient phrenic nerve palsy, but all recovered prior to discharge. Due to the proximity of the CTI to the right coronary artery, several patients who underwent additional CTI ablation developed coronary artery spasm, which was effectively treated with intracoronary nitroglycerin.22,50
Limitations
This study has several limitations that warrant consideration. First, the follow-up duration of the studies included was up to 12 months, with a mean follow-up duration of 8.6 months. Very late recurrence (>12 months after ablation) is more common than previously anticipated. Long-term follow-up studies show that the longer the follow-up period after ablation, the greater the recurrence rate, with PV reconnection being the primary cause of late recurrence.51,52 This is further demonstrated by the trend of increased recurrence in trials with a longer follow-up (Supplementary Figure 1D), despite the fact that PVI durability and AF recurrence measures were comparable between the two groups. Therefore, longer-term results are needed to corroborate our findings. Second, most patients had paroxysmal AF, and because outcomes were better for paroxysmal AF than persistent AF, this may have contributed to significant discrepancies in the outcomes of interest. Third, most studies used a FARAWAVE catheter; thus, the results cannot be generalised to other types of catheters. Fourth, randomised controlled trials comparing PFA to thermal ablation are lacking. Finally, the fact that all the patients in the included studies were White and from European nations reduces the generalisability of our findings, underlining the need for further validation in other ethnic groups.
Conclusion
PFA is associated with a shorter procedural time than thermal ablation. Cardiac complications were rare after both PFA and thermal ablation, and mostly reversible in nature, with no significant difference between the two groups. More prospective randomised controlled trials with longer follow-up periods are needed to verify our findings.
Clinical Perspective
- PFA had a better overall procedure time and a longer fluoroscopy time than thermal ablation for PVI of AF, but AF recurrence rates, PVI durability and periprocedural complications were comparable between the two groups.
- Adverse events and complications were rare in both the PFA and thermal ablation groups, with most appearing to be preventable.
- Additional ablation beyond PVI in PFA may not provide additional benefits in addition to PVI alone, although this has yet to be proven with further longer-term investigations, particularly in the persistent AF subgroup.