Since the first catheter ablation for cardiac arrhythmia more than three decades ago, ablation technology has continually evolved at a rapid pace. Much of the early progress in the field was made in ablation of supraventricular tachycardias. Following a seminal study from Haïssaguerre et al.1 in 1998, which demonstrated that pulmonary vein triggers are important sources of atrial fibrillation (AF), the approach to management of AF underwent a revolution. Electrical isolation of pulmonary veins (PVs) using catheter ablation became an established therapeutic strategy in patients with paroxysmal AF. During subsequent years, the role of ablation in AF expanded, and more extensive strategies involving ablation of non-pulmonary vein triggers and modification of the left atrial substrate were demonstrated to be effective, even in persistent forms of AF.2
In recent years catheter ablation has also emerged as an effective treatment strategy for patients with ventricular tachycardia (VT). An important area of expansion is the use of catheter ablation for treatment of recurrent VT in the context of ischaemic cardiomyopathy (ICM) or non-ischaemic cardiomyopathy (NICM). VT ablation is commonly used in ICM and NICM patients who have recurrent defibrillator shocks due to drug-refractory VT. Many of the technological advances in AF ablation have been used to develop ablation techniques for scar-related VT.
In parallel with the expanding role of catheter ablation for AF and VT, multiple novel technologies have been developed to simplify the procedures while at the same time aiming to increase safety and procedural success. The aim of the present review is to provide an overview of novel developments in AF ablation and VT ablation in the context of structural cardiac diseases. Ablation of other supraventricular tachycardias and VT in the context of structurally normal hearts has previously been reviewed extensively and is not discussed here.
New Technologies and Techniques for AF Ablation
Currently, the most widely used technique for PV isolation involves delivery of point-by-point ablation lesions around the circumference of the vein. A number of different variations to the approach have been developed. During the early stages of PV isolation, a ‘segmental approach’ which involved targeting the earliest PV potentials at the ostium of the PV was commonly used. Due to high reconnection rates and the risk of PV stenosis, the technique has progressively been modified and the prevailing technique involves circumferential antral ablation to achieve PV isolation.3
(Film demonstrating ablation of ventricular tissue in a sheep model under direct visualisation using the IRIS catheter. Following delivery of radiofrequency energy, blanching of the tissue is seen, indicating delivery of an effective ablation lesion)
Techniques for modification of the left atrial substrate for AF include linear ablation and ablation of complex fractionated electrograms. These techniques are more widely used in patients with persistent AF as an adjuvant strategy to PV isolation.3 Both techniques conventionally involve point-by-point ablation. The aim of linear ablation is to divide the atrium into smaller segments which are less likely to sustain macroreentrant arrhythmias.3 The most common sites of linear ablation are the left atrial roof and the mitral isthmus region. Ablation of complex fractionated electrograms, which may be representative of ´rotors´ that drive AF, involves targeting fractionated areas with short cycle lengths. It is important to note that the relationship between fractionated regions and rotors remains speculative.
Advances in Catheter Design for AF Ablation
A point-by-point approach for AF ablation is associated with a number of limitations, including prolonged procedural times. Therefore novel catheter designs, which allow simultaneous application of multiple ablation lesions around the circumference of the PVs or in the left atrium, have been developed. Examples include balloon-mounted ablation techniques and multi-electrode catheters.
Balloon-mounted technologies focus on PV-trigger dependent AF which is mostly observed in patients at early stages of paroxysmal AF. Three different balloon-based technologies have been used to ablate PV ostia; cryoablation, high intensity ultrasound and laser.2 These ablation systems are designed to either ablate the entire ostium of the pulmonary vein or certain arcs of the pulmonary vein circumference.2 Initially there were reports of limited success with balloon-based techniques due to their inability to ablate non-PV sites and technical challenges associated with isolation of the right inferior pulmonary vein. However, more recent studies have reported that these techniques have comparable success rates with RF ablation for PV isolation and shorter procedure times.4–7
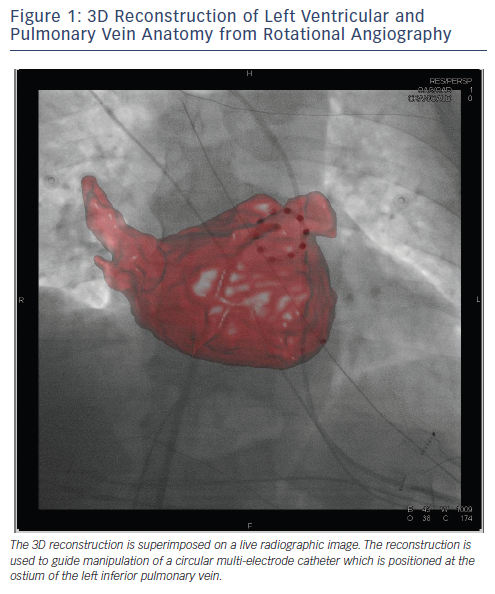
Multi-electrode ablation catheters are another technology for simultaneous delivery of multiple ablation lesions during AF ablation. Early multi-electrode designs include the MESH® catheter (Bard Electrophysiology, MA, USA) and the Pulmonary Vein Ablation Catheter® (PVAC) (Medtronic Ablation Frontiers, CA, USA). The MESH catheter is an expandable non-steerable circular catheter with 36 electrodes.2 The PVAC is a circular deflectable catheter with 10 poles capable of delivering RF energy in unipolar and bipolar modes.2 One of the major limitations of these catheter designs is the lack of irrigation. In an attempt to overcome this limitation, the nMARQ™ catheter (Biosense Webster, CA, USA), which is an irrigated multipolar catheter, has recently been developed. Studies are ongoing to determine longterm outcomes following ablation with the nMARQ catheter (see Figure 1).4
In addition to their role in PV isolation, multi-electrode catheters have been developed for substrate-based ablation in the left atrium. The Tip-Versatile Ablation Catheter (TVAC; Medtronic Ablation Frontiers, CA, USA) has been designed to create simultaneous linear lesions in the left atrium e.g. roof lines, mitral isthmus lines and cavotricuspid isthmus lines.8 The TVAC has previously been reported to have comparable outcomes to conventional ablation for cavotricuspid isthmus lines with reduced procedure times.8 There are currently no randomised studies comparing conventional ablation with TVAC for roof and mitral lines.
One of the most important recent developments in AF ablation is the design of catheters that provide feedback on contact force during ablation. These catheters have sensors integrated into the tip which provide real-time information on the force of contact. A number of studies have convincingly demonstrated that catheter contact force correlates with the delivery of effective ablation lesions and durable PV isolation.9–12 Further, clinical outcomes have been reported to be superior in patients undergoing AF ablation with contact force catheters as compared with conventional ablation catheters.13 The two main contact force catheters currently in use for AF ablation are the ThermoCool© SmartTOUCH™ catheter (Biosense Webster, CA, USA) and the TactiCath™ catheter (Endosense, Inc., Geneva, Switzerland).
Remote Navigation Technologies for AF Ablation
Remote navigation technologies have been developed in recent years to simplify catheter manipulation during AF ablation.4 The three main remote navigation technologies include the Niobe® magnetic navigation system (Stereotaxis Inc., MO, USA), the Sensei™ robotic navigation system (Hansen Medical, CA, USA) and the Amigo™ remote catheter system (Catheter Robotics Inc., NJ, USA). The three systems use different technologies to allow remote navigation. While the Niobe system uses a remote magnetic system, the other two systems use remote catheter manipulators. The overall effect is that operators can manipulate catheters from a distance using a 3D navigation handle.14 Potential advantages of these technologies include increased safety, more precise catheter manipulation and increased stability.15 A number of studies have demonstrated that the results of PV isolation with remote navigation are comparable with conventional ablation techniques.16,17 However, they are also associated with drawbacks, the most important of which relate to the cost and logistical aspects of installation of the technology.
Advances in Imaging Techniques for AF Ablation
During the early stages of AF ablation, catheter navigation was based solely on fluoroscopic guidance and intracardiac signals. AF ablation was therefore associated with significant radiation doses and occasionally difficulty in determining catheter orientation.4 The emergence of electro-anatomical mapping (EAM) techniques has been a major development in the field. EAM systems are designed to create a 3D geometry of the left atrium and PVs and allow precise localisation of the catheter tip within the model.4 Further, these systems allow for identification of scar and provide information on electrical activation relative to the anatomical map. An added advantage is that EAM allows operators to identify areas of incomplete ablation.4,18
The two most commonly used EAM techniques are the Carto® system (Biosense Webster, CA, USA) and the EnSite™ NavX™ system (St Jude Medical, MN, USA). Since their conception, EAM techniques have continued to evolve, and current iterations allow for integration of data from 3D reconstructions from computerised tomography (CT), rotational angiography and magnetic resonance imaging (MRI) scans. As a result, it is possible to delineate complex left atrial and PV anatomy with a high degree of accuracy.2,19,20 More recently, novel mapping systems such as the Rhythmia™ mapping system (Boston Scientific Inc., MA, USA) have been demonstrated to rapidly generate high-resolution maps in animal models.21
MRI with late gadolinium enhancement has emerged as a valuable technique for identifying regions of atrial fibrosis and scarring. The degree of fibrosis has been demonstrated to predict outcome in patients undergoing AF ablation.22 In the future, MRI may play a significant role in patient selection for AF ablation. Further, the recent development of MRI-compatible catheters has opened up a new area of research. Early studies have demonstrated that real-time MRI can be used to guide catheter placement.23
Rotational angiography is a potentially valuable tool for real-time imaging in patients undergoing AF ablation. Rotational angiography involves real-time acquisition of left atrial and PV anatomy after injection of contrast in the atrium. Images are then reconstructed superimposed on real-time fluoroscopic images (see Figure 1).19,20,24 It is also possible to integrate rotational angiography images with electroanatomical maps. A number of rotational angiography technologies are currently available including EP Navigator (Philips Healthcare, Best, The Netherlands) and DynaCT Cardiac (Siemens, Forchheim, Germany). Potential advantages of rotational angiography over EAM systems include less anatomical distortion due to more rapid creation of left atrial geometry.4,25
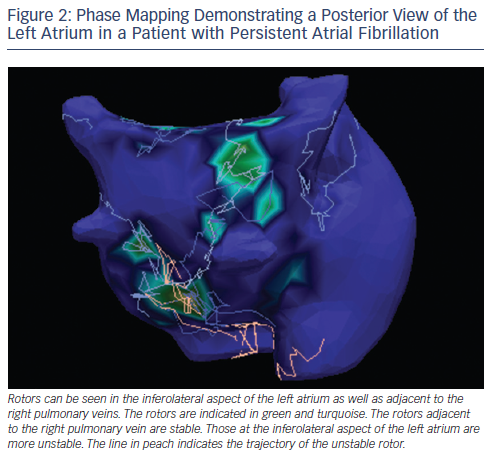
A novel technology that could potentially revolutionise management of AF and especially left atrial tachycardia and flutter is electrocardiographic imaging (ECGI). The technique utilises more than 250 electrodes positioned on the torso to record unipolar electrograms from the atrial epicardial surface. CT scanning is used to determine the atrial anatomy and the positions of the electrodes relative to the atrium.26 The recorded unipolar electrograms are used to derive information on cardiac activation patterns using mathematical modeling. A number of recent studies have demonstrated promising results using ECGI. Shah et al. reported that in 44 patients with atrial tachycardia ECGI (ECVUE mapping system, CardioInsight Technologies Inc., OH, USA) effectively localised the source of atrial tachycardia in 100 % of patients. Further in 92 % of cases, the mechanism of atrial tachycardia was accurately diagnosed.27 In a feasibility study by Haissaguerre et al., ECGI was demonstrated to identify active sources of AF with high resolution.28 Specifically, they demonstrated active sources in the vicinity of the pulmonary veins in patients with paroxysmal AF and more widespread sources in patients with the more sustained form of the arrhythmia. A number of additional studies have also used non-invasive mapping to identify AF sources which have been targeted for ablation.29,30 An example of rotors identified by ECGI is included in Figure 2. ECGI-based ablation is currently in the investigational phase, and multicentre trials are ongoing to determine the efficacy of the technique.
New Technologies and Techniques for Ablation of Ventricular Arrhythmias
During the early stages of VT ablation, the ablation strategies were based primarily on classical techniques such as entrainment and activation mapping to target the critical isthmus of the VT circuit.31,32 While these techniques are effective in a proportion of VT cases, they are associated with significant limitations. Most importantly, they are reliant upon the ability of the operator to induce clinically relevant sustained tachycardias that are haemodynamically tolerated. Due to these limitations, substrate-based ablation techniques have become increasingly prominent. Substrate-based ablation involves targeting late and fractionated electrograms that are suggestive areas of scar and abnormal conduction during sinus rhythm.33 The arrhythmogenic substrate may be endocardial, epicardial or both.
Advances in Imaging Techniques for VT Ablation
Scar-related VT ablation is critically dependent upon detailed delineation of ventricular anatomy and localisation of the scar and border zone. EAM is used extensively for these purposes in VT patients.34 As discussed previously, EAM systems create 3D chamber geometry as well as identifying areas of abnormal voltage, and hence scar.4 EAM systems can be used to create both epicardial and endocardial scar maps during VT ablation. It is important to note that while EAM is considered as the standard imaging modality for VT ablation, it is associated with limitations. For instance, single voltage measurements are unlikely to provide accurate representation of complex, 3D intramural scars. Further, EAM is associated with a risk of incorrectly identify areas of low voltage due to poor contact.35,36
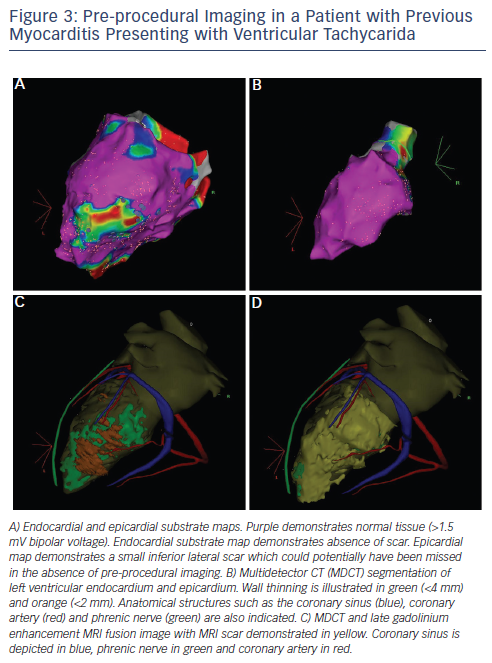
Delayed enhancement MRI (DE-MRI) and multidetector CT (MDCT) imaging have emerged as valuable adjunctive techniques that may overcome some of the limitations of using EAM in isolation. As is the case in AF patients, DE-MRI and MDCT images can be integrated with EAM maps. DE-MRI provides high-resolution 3D imaging of scar size, location, heterogeneity and transmurality. Multiple studies have demonstrated that areas of delayed enhancement correlate with low voltage areas on EAM (see Figure 2).37–39 Delayed enhancement is correlated with sites of successful ablation in ICM patients.39 Further, DE-MRI has been reported to identify slow conduction channels that are potentially important regions of VT circuits.40 It is important to note however that, in the majority of centres, DE-MRI is currently restricted to patients who do not have an implantable cardioverter defibrillator (ICD). The development of MRI-compatible ICDs is predicted to significantly expand the role of this imaging technique in VT ablation.
MDCT is associated with high spatial and temporal resolution.41 MDCT is effective for identifying areas of ventricular calcification, fibro-fatty replacement, wall thinning and epicardial fat. Areas of wall thinning have been demonstrated to correlate with low voltage areas identified during EAM (see Figure 2).42 Further, MDCT has been demonstrated to identify areas harbouring local abnormal ventricular activity (LAVA) which, as discussed in subsequent sections, is critical to the VT mechanism.43 A major advantage of MDCT over DE-MRI is that the technique can be used for imaging of patients with an ICD. Additional advantages of MDCT include delineation of the coronary arteries, the phrenic nerve and papillary muscle.44 Pre-procedural annotation of these structures is important for minimising intraprocedural risk. Further, accurate localisation of epicardial fat using MDCT makes epicardial voltage mapping more reliable. Overall, DE-MRI and MDCT provide complementary information on the arrhythmogenic substrate in patients undergoing VT ablation.44
Recently, ECGI has also been investigated as a potential additional imaging modality for mapping of VT. Wang et al. demonstrated that ECGI accurately identifies the site of origin of VT in more than 90 % of cases when compared with invasive mapping.45 Further, ECGI identified the mechanism of VT with a high degree of accuracy. Therefore, in addition to the expanding role in atrial arrhythmia, ECGI may emerge as an effective tool for mapping of VT. While research into the role of ECGI in VT is at an early stage, the technique has the potential to provide valuable information that can be used for pre-procedural planning of the ablation strategy. It is important to note however that, at this stage, the role of ECGI in patients with scar-related VT is not clear, and further research is necessary to validate its role in this context.
Advances in VT Mapping Techniques
As discussed in the previous section, EAM is the most widely used imaging modality during ablation of scar-related VT. EAM commonly involves point-by-point sampling using conventional bipolar catheters. However this approach is time consuming, and mapping density is often inadequate. As a result, a number of novel multipolar mapping technologies have been developed to facilitate rapid and highdensity activation mapping. Examples include microelectrode ‘basket’ catheters, non-contact microelectrode arrays and multipolar catheter such as Pentaray and duodecapolar catheters.
Microelectrode ´basket´ catheters have an expandable design with multiple splines, which are designed to conform to the shape of the cardiac chamber. Each spline contains multiple recording electrodes.34 The Constellation® basket catheter (EP Technologies, CA, USA), has previously been reported to significantly reduce mapping times in patients with scar-related VT.46,47 However, these catheters are associated with multiple potential limitations. For instance, inadequate deployment of the splines can result in incomplete mapping. Further the catheter can interfere with ablation catheter manipulation and potentially cause mechanical trauma.34 Overall the use of basket catheters for VT ablation has been limited to small case series.34
Non-contact microelectrode arrays consist of inflatable balloons with multiple unipolar electrodes on the surface. The electrodes are designed to detect far-field electrical potential in addition to the location of a roving standard mapping catheter.34,48 Movement of the roving catheter within the ventricle is used for construction of endocardial geometry. Inverse solution mathematics is used to superimpose numerous reconstructed electrograms on an endocardial model.49 These systems are designed to provide detailed endocardial mapping during a single beat.48 Non-contact mapping is primarily designed for activation mapping and, given that it can map activation with a single beat, it may be of use in patients with poorly tolerated VT. Overall however, while non-contact systems have been used for mapping of scar-related VT, their utility is not widespread.50–52
Steerable multipolar catheters have also been developed for high-density mapping during VT. Examples include the Livewire™ duodecapolar catheter (St Jude Medical, MN, USA) and the PentaRay® catheter (Biosense Webster, CA, USA).33,53 The duodecapolar catheter is a 20 electrode steerable catheter. Two previous studies have demonstrated that the catheter can be used can be used to acquire high-density maps of the epicardial and endocardial surfaces.53,54 The PentaRay catheter consists of five soft and flexible splines with multiple electrodes on each spline. The catheter is designed to minimise traumatic complications during endocardial and epicardial mapping. A major advantage of the PentaRay catheter in the context of VT mapping is that, in addition to endocardial mapping, it can be used to acquire high-density maps of the epicardial surface. Jais et al. demonstrated that the PentaRay catheter produces minimal ectopy during epicardial mapping,33 and is associated with minimal artificial signals. Therefore, during endocardial VT ablation, the PentaRay catheter can be used to monitor transmural response.
Advances in VT Ablation Strategies
As discussed previously, VT ablation using activation and entrainment mapping has traditionally been the most widely used strategy for VT ablation.55 However, a major limitation of these approaches is that they depend upon induction of monomorphic VT which is clinically relevant and well tolerated. As a result of these limitations, substrate-based approaches have been used increasingly in VT patients. Strategies for substrate-based ablation include linear ablation across voltage channels, encircling of scars, and homogenisation of regions of heterogenous scar.
It is important to note that substrate-based approaches are also associated with challenges. One of the major challenges is the definition of the endpoint following ablation. Non-induciblity of VT has been used as an endpoint by many operators. However, this approach is associated with important limitations including non-reproducibility and lack of compelling data to suggest that non-inducibility predicts long-term outcome. Overall, there is currently no general consensus as to the optimal endpoint of substrate-based VT ablation.
Recently, ablation of LAVA has become an increasingly prominent substrate-based ablation technique.33,56–58 The aim of LAVA ablation is the dissociation or isolation of surviving myocardial fibres within scar regions.33 Importantly, the endpoint of LAVA-based ablation is complete LAVA elimination. Therefore, this approach overcomes the aforementioned limitations of non-inducibility of VT as an endpoint. Jaïs et al. recently demonstrated that complete elimination of LAVA is safe and is associated with a superior clinical outcome.33 More recently, the same group demonstrated that in ICM patients with secondary wall thinning, epicardial LAVA can be eliminated with an endocardial approach, thereby limiting the amount of epicardial ablation.59
Pace- mapping provides valuable information during substrate-based VT ablation. Pace-mapping involves pacing during sinus rhythm at different sites and comparing the activation sequence with that of the clinical VT. Automated algorithms can be used for comparison of QRS morphologies. While pace-mapping is commonly used as an adjunctive technique during scar-related VT ablation, it is associated with important limitations. For instance, in addition to providing pacemaps that match the clinical VT at the VT exit site, normal tissue can also produce matching pace-maps due to large reentry circuits.34 In an interesting recent study however, De Chillou et al. demonstrated that, in patients with ICM, performing high density pace-mapping and annotation using an EAM system can accurately identify the entry and exit points of a VT circuit as well as demonstrating the orientation of the critical isthmus.60 Further, they were able to demonstrate bidirectional block across the isthmus following linear ablation.
Advances in Ablation Techniques for VT
One of the major contributors to VT recurrences in patients with scar-related VT is the inability to create adequate lesions in areas critical to the VT circuit. Deep intramural VT circuits are particularly challenging in this context. Intramural VT circuits may be inaccessible to ablate with epicardial and/or endocardial approaches. A number of technologies have therefore been developed in an attempt to overcome these limitations. Examples include transcoronary ethanol injection, bipolar ablation, needle-based catheters and catheters that allow direct visualisation of myocardial tissue. These techniques are discussed in more detail below.
Transcoronary ethanol ablation for VT has been in existence for more than two decades.61 The technique involves identification of the branch of the coronary tree supplying the arrhythmogenic substrate and injecting ethanol to ablate the substrate. Initial strategies for selection of coronary branches were based primarily on anatomical considerations. Over the years, the procedure has been refined to more accurately define the coronary branches of interest. For instance, pace mapping with angioplasty guide wires in the coronary circulation has been demonstrated to effectively guide transcoronary ablation. A number of recent studies have demonstrated that, in patients with difficult-to-control VT despite radiofrequency ablation, transcoronary ethanol ablation is an effective alternative strategy. It is important to note however that efficacy of this technique is limited by factors such as unfavourable coronary anatomy and recurrence of modified VT.62
High-power bipolar ablation is a potentially effective technique for ablation of deep intramural VT circuits, particularly circuits arising from within the septum. Bipolar ablation involves positioning two catheters on either side of the septum or endo- and epicardially and delivering high-power radiofrequency energy. In animal infarct models, and more recently in explanted ex vivo human hearts, bipolar ablation has been demonstrated to more effectively create transmural lesions as compared to standard unipolar ablation.63,64 The technique has also been demonstrated to be effective in case reports and small series of patients with VT which is refractory to conventional ablation techniques.65,66
An interesting novel technique designed to reach deep intramyocardial arrhythmogenic substrates is needle-based catheter ablation.67 The catheter design has a needle tip which can be expanded and retracted. The needle tip is irrigated and can map as well as ablate. The technique involves perforation of the myocardium with the needle and delivery of energy to create deep intramural lesions. In a recent feasibility study, the catheter demonstrated promising results.67 However the technique is currently in the investigational phase and further research is warranted to more clearly define its role in VT ablation.
Finally, catheters that allow direct visualisation during ablation have demonstrated promising results in animal models. Sacher et al. demonstrated that the IRIS™ catheter (Voyage Medical Inc., CA, USA), which allows direct visualisation during ablation, reliably created ablation lesions in 99 % of application sites with minimal complications in a sheep model. Further, the catheter was significantly more effective when compared with a standard open-irrigated tip catheter in creating ablation lesions.68 Once again, this technology is currently in the research phase and studies in humans have not been conducted.
Conclusions
Catheter ablation of cardiac arrhythmias is a constantly expanding and evolving field. In recent years, advances in catheter ablation techniques have significantly improved outcomes in patients with AF and VT. However, these techniques remain time consuming and in a proportion of patients, ineffective. Therefore, there remains a need for continual technological advances to improve outcomes.







