Atrial fibrillation (AF) is the most frequently encountered arrhythmia in clinical practice and has become an emerging epidemic. AF is associated with increased cardiovascular mortality and morbidy such as stroke.1–3 Over 2.3 million people in the US are affected by AF: it is estimated that AF will affect more than 15 million Americans by 2050.3 The traditional risk factors implicated in the pathogenesis of AF include age, hypertension, diabetes, obesity, coronary artery disease (CAD) and congestive heart failure.4,5 Recent studies revealed that the prevalence of obstructive sleep apnoea (OSA) is substantially higher among patients with AF (ranging from 32 to 49 %), strongly indicating that OSA may be contributing to the initiation and progression of AF.6,7
Sleep apnoea, a severe form of sleep-disordered breathing, is broadly divided into two categories: central sleep apnoea (CSA) and OSA.8 CSA is caused by abnormal responses in the brain stem that controls the respiration drive, leading to the Cheyenne-Stokes pattern of respiration. CSA is one of the most common comorbidities in patients with heart failure. Cheyenne-Stokes respiration, commonly observed in CSA patients, is caused by a complex interaction among increased pulmonary capillary/venous pressure, fluctuation of blood oxygen and CO2 level, and chemoreceptor function. The incidence of CSA in heart failure patients ranged from 21 to 82 %, depending on the severity of heart failure and the cut-off value of the apnoea–hypopnoea index (AHI) adopted in different studies.9 The severity of CSA also correlates with the incidence of arrhythmias such as AF.
OSA is caused by obstruction of the upper airway despite increased efforts of breathing exerted by the thoracic and abdominal respiratory muscles. OSA affects approximately 24 % of men and 9 % of women, between 30 and 60 years of age.8,10,11 It is estimated that approximately one in 15 adults has at least moderate OSA and most cases remain undiagnosed.8,10,11 The incidence of OSA has increased progressively among people of different ages. OSA induces intermittent hypoxia, hypercapnia, intrathoracic pressure shifts, hyperactivity of the autonomic nervous system and abrupt surges in arterial pressure and inflammation, leading to hypertension, diastolic dysfunction, left atrial enlargement and atrial fibrosis. All of these diseases are established risk factors or contributing factors to AF.5–8
Definition and Diagnosis of Central Sleep Apnoea and Obstructive Sleep Apnoea
Based on the high prevalence of sleep apnoea, particularly CSA, in the heart failure patients, it is advisable to ask the patient’s spouse or partner about any abnormal respiratory pattern during sleep. When a new arrhythmia such as AF is diagnosed in a heart failure patient, screening for sleep apnoea is worthwhile. OSA can be implicated on the basis of medical history (e.g. snoring, witnessed apnoeas, waking up with a choking sensation, and excessive daytime sleepiness) and physical examination (e.g. short neck, increased neck circumference).12 The gold standard of diagnosing sleep apnoea is overnight polysomnography, which measures the air flow, respiratory muscle activity, electroencephalography, electrocardiogram (ECG) and blood pressure (BP). CSA can be distinguished from OSA by the absence of abdominal or thoracic respiratory muscle efforts during the apnoeic episode. Apnoea is defined as airflow reduced to less than 10 % of baseline for more than 10 seconds. Hypopnea is defined as a reduction of airflow to less than 50 % of baseline for more than 10 seconds, in association with a ≥3 % oxygen desaturation or arousal from sleep. Severity of sleep apnoea is measured by the AHI and the frequency of apnoeas and hypopnoeas per hour of sleep. An AHI of 5–15 is considered mild, while severe sleep apnoea is defined as AHI ≥30.12–16
The growing awareness of sleep apnoea among physicians inevitably leads to a long waiting period for an overnight polysomnograpy study. Unattended portable monitoring, with a cost only 10–20 % of an overnight polysomnography study, has emerged as a screening tool for OSA. These portable devices are capable of recording respiratory movement, airflow and blood oxygenation. More advanced devices also provide an ECG/heart rate channel for heart rhythm monitoring.17 While portable monitoring tends to underestimate the AHI score in patients with heart failure and chronic obstructive pulmonary disease, portable monitoring may to evolve to a diagnostic tool for patients with high clinical suspicion of OSA in which a positive test essentially verifies the diagnosis of OSA without the need for an overnight polysomnography study.
Association between Atrial Fibrillation and Obstructive Sleep Apnoea
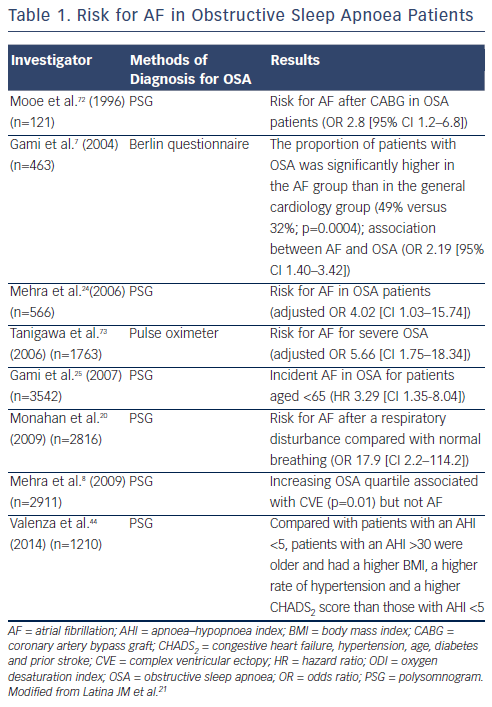
Multiple studies have demonstrated that AF is substantially more prevalent in patients with OSA than those without OSA.18–21 The frequency of arrhythmias increases with the severity of the OSA. Table 1 summarises the studies showing an increased risk for AF in OSA patients. One of the early studies that analysed the prevalence of cardiac arrhythmias and conduction disturbance in 400 OSA patients was performed using 24-hour Holter monitoring. Guilleminault et al.22 found a slight nonstatistically significant increase in the prevalence of paroxysmal AF in this group of patients. However, most studies identified a much higher prevalence of AF in OSA patients. For example, in a prospective sleep study, Hoffstein et al.23 followed 458 subjects with suspected OSA undergoing polysomnography. The authors reported a 58 % prevalence of arrhythmias in those with OSA (AHI >10) and 42 % in the controls without OSA (AHI ≤10; p<0.001). In addition, the author also found that the rate of cardiac arrhythmias increased with AHI, with 70 % of individuals (AHI ≥40) having arrhythmias versus 42 % of individuals with an AHI ≤10 (p=0.002). In two groups of patients sampled from the study population of the Sleep Heart Health Study, 228 patients with a high sleep-disordered breathing index (respiratory disturbance index [RDI] ≥30) and 338 subjects with a low index (RDI <5), Mehra et al.,24 discovered that the risk for AF in patients with severe OSA is about four times higher than those without OSA (adjusted odds ratio [OR]=4.02, 95 % confidence interval [CI] 1.03–15.74). Gami et al.25 conducted a retrospective cohort study of 3,542 Olmsted County adults with OSA diagnosed by polysomnogram. New-onset AF was confirmed by electrocardiography during a mean follow-up of 4.7 years. The authors discovered that AF occurred in 133 subjects (cumulative probability 14 %). Univariate predictors of AF include age, male gender, hypertension, CAD, heart failure, smoking, body mass index, OSA (hazard ratio [HR] 2.18), and the severity of OSA.
While OSA patients have a higher incidence of AF, it has also been shown that OSA is substantially more prevalent in AF patients as well. Except for a few studies showing the lack of increased incidence of OSA in AF patients, the majority of studies demonstrated otherwise.26,27 For example, Gami et al. prospectively studied consecutive patients undergoing electrocardioversion for AF (n=151) and consecutive 312 patients referred to a general cardiology practice without a past or current history of AF (n=312).7 They showed the proportion of patients with OSA was significantly higher in the AF group than in the general cardiology group (49 % versus 32 %; p=0.0004). The adjusted OR for the association between AF and OSA was 2.19 (95 % CI 1.40–3.42; p=0.0006).
Stevenson et al. also demonstrated similar results in 90 patients with paroxysmal or persistent AF and 45 controls.28 They found that AHI in AF patients was higher than in controls (23.19±19.26 versus 14.66±12.43; p=0.01). The OR for the association between AF and sleep disordered breathing (SDB) (AHI >15) was 3.04. Bitter et al. reported similar findings in which sleep apnoea was documented in 74 % of all patients with AF (43 % had OSA and 31 % had CSA).29
Mechanisms Underlying Atrial Fibrillation in Obstructive Sleep Apnoea
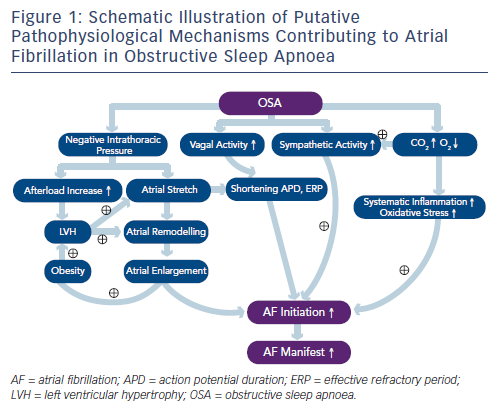
OSA is characterised by sleep-related periodic breathing and repetitive collapse of the upper airway, resulting in hypoxia, hypercapnia, sleep arousals, shifts in intrathoracic pressure, and hyperactivity of the autonomic nervous system.30–32 AF and OSA share some common risk factors, such as advanced age, obesity, male gender, hypertension and CAD.30–33 It seems that several pathophysiological mechanisms may account for both OSA and AF; therefore, the presence of one may promote the other. Studies also demonstrated that OSA may promote cardiac remodelling and systemic inflammation.31–35Figure 1 summarises potential mechanisms that may be responsible for the initiation and maintenance of AF in OSA patients.
Hypoxia, Oxidative Stress and Inflammation
OSA induces repeated episodes of hypoxia and hypercapnia that trigger chemoreflex and enhance the sympathetic nerve activity, leading to tachycardia and BP surges, especially at the end of the apnoeic episodes.22,35 Tachycardia and hypertension increases myocardial oxygen demand while myocardial oxygen supply is at its lowest level due to hypoxia. This results in repeated myocardial ischaemia during sleep, promotes atrial and ventricular fibrosis and subsequently induces atrial and ventricular arrhythmias as well as sudden cardiac death during sleep.34,35
Intermittent hypoxia and post-apnoeic re-oxygenation lead to excessive oxidative stress, which also plays a major role in inflammation.35–40 Repetitive oxidative stress on the myocardium may result in adverse myocardial remodelling and inflammation, thereby producing a substrate for AF. Shamsuzzaman et al. reported significantly higher levels of plasma C-reactive protein (CRP) in patients with OSA than in control subjects (0.33 versus 0.09 mg/dl) and CRP levels were independently associated with the OSA severity as well.36 In addition, systemic oxidative stress was markedly elevated in patients with OSA than the controls.37,38 Treating OSA with continuous positive airway pressure (CPAP) significantly attenuated the effect of OSA on CRP and interleukin (IL)-6 levels.39 Inflammation and oxidative stress also leads to vascular endothelial dysfunction, predisposing OSA patients to atherosclerosis.40
Substantially Negative Intrathoracic Pressure
OSA is characterised by repetitive forced inspiration against an obstructed upper airway that generates a substantially negative intrathoracic pressure (e.g. –65 mmHg) with a subsequent increase in the left ventricular (LV) transmural pressure (afterload).41,42 Increased afterload can lead to LV hypertrophy. Orban and colleagues performed the Mueller manoeuvre to simulate OSA in 24 healthy young adults.43 The Mueller manoeuvre involves a forced inspiration against a closed mouth and nose in order to make a substantially negative pressure in the chest. They found that left atrial volume markedly decreased and LV end-systolic dimension increased with a decreased LV ejection fraction during the manoeuvre. After releasing the manoeuvre, there was a compensatory increase in blood flow, stroke volume, ejection fraction and cardiac output exceeding baseline. They proposed that repetitive swings in afterload burden and chamber volumes may have implications for future development of AF and heart failure. In addition, overly negative intrathoracic pressure is transmitted to the thin-walled atria and leads to atrial stretch. Repeated stretch may result in atrial chamber enlargement and fibrosis, both of which are known to predispose atria to AF.41–44 It has been suggested that negative tracheal pressure during obstructive apnoea is a strong trigger for AF, which was mediated by shortening the atrial effective refractory period (ERP) and increasing susceptibility to AF mainly by enhanced vagal nerve activity.41,42 Obstructive Sleep Apnoea and Atrial Remodelling Atrial structural remodelling and electrical remodelling are well known to be critical elements in the pathogenesis of AF. Importantly, several studies have demonstrated that OSA may increase left atrial size independently, leading to atrial conduction abnormalities, and longer sinus node recovery time (SNRT) in both animal and human studies.45–48 Animal studies demonstrated that AF induced by OSA is related to shortening of the ERP.42,47,49 Using electroanatomical mapping, patients with OSA showed significant atrial remodelling including atrial enlargement, voltage decrease, conduction abnormalities and longer SNRT.34 In a consecutive group of 720 AF patients undergoing cardiac magnetic resonance imaging before AF ablation, Neilan et al. reported that patients with OSA have an increased BP, right ventricular volume, left atrial size and LV mass.50 All these studies suggest that OSA is associated with atrial structural and electrical remodelling characterised by atrial enlargement and reduction in voltage, as well as conduction abnormalities. Treatment with CPAP is associated with lower BP, decreased atrial size, and ventricular mass, and a lower risk for AF recurrence after pulmonary vein isolation (PVI).50
Hyperactivity of the Cardiac Autonomic Nervous System
Normal sleep is often divided into nonrapid eye movement (NREM) sleep and rapid eye movement (REM) sleep. When a person falls asleep, the NREM sleep progresses from stage 1 to stage 4 before entering the REM stage. The cycles of NREM and REM sleep repeat themselves throughout the period of sleep.11,51–53 During NREM sleep, there is a progressive increase in the parasympathetic tone and withdrawal of sympathetic tone, manifesting as slowing of the heart rate, reduction of BP and a decrease in the sympathetic nerve activity.53 When the REM sleep begins, there is a surge of the sympathetic nerve activity, BP and heart rate.53–55 This pattern of natural variations in the sympathetic/parasympathetic balance is disturbed in patients with OSA. For instance, the timing of sudden death in the general population is known to be highest between 06:00 and noon when the sympathetic tone is high. In patients with OSA, the highest incidence of sudden death occurred between midnight and 06:00 when the victims were in sleep.56 In addition, in patients with OSA, because of the nightly struggle with respiration, the baseline sympathetic activity is significantly higher than those without OSA, leading to increased risks for cardiovascular diseases and metabolic disorders.15,57 Moreover, repetitive hypoxia and hypercapnia stimulate the central and peripheral chemoreceptors that augment sympathetic nervous activity.58 At the same time, baroreflex in OSA patients is attenuated, leading to unopposed sympathetic activation, resulting in marked vasoconstriction and hypertension.
Previous experimental studies have shown a close mechanistic association between the autonomic nervous system and AF induced by OSA.41,42,47,49 In a canine model of OSA, direct neural recording from a ganglionated plexi (Ao-SVC GP), located at the junction of the aorta, superior vena cava and right pulmonary artery, revealed markedly
increased neural activity preceding the initiation of AF. Ablation of the Ao-SVC GP, which was proposed to be the gateway for vagal innervation to the heart, markedly suppressed AF inducibility in this canine model of OSA. Linz et al. reported that in a pig model of OSA, the negative intratracheal pressure generated by forced inspiration, known to activate afferent vagal fibres in the thorax, activated the parasympathetic nervous system, which in turn facilitated the initiation of AF.49,59 Airway obstruction without generating a strong negative intratracheal pressure failed to initiate AF. AF initiation was also inhibited by vagotomy. Acute atrial stretch induced by markedly increased intrathoracic pressure generated by forced inspiration as well as diastolic dysfunction in obese animals also play major roles in the genesis of AF in OSA.45,48
Initiation and Maintenance of Atrial Fibrillation in Obstructive Sleep Apnoea Patients
As discussed above, surges of sympathetic and parasympathetic activity are induced by OSA.42,47 The former induces a large and extended Ca++ transient and the latter markedly shortens the action potential duration and refractory period, leading to triggered firing of the PV and atrium, thereby inducing AF.60 Atrial stretch, caused by a substantial drop of the intrathoracic pressure in order to compensate for the obstructed airway, markedly shortens the atrial and PV refractory period, similar to the electrophysiological effect of parasympathetic activation.61,62 Prolonged atrial conduction time, increased dispersion of the refractoriness and enhanced atrial fibrosis caused by repeated OSA all contribute to the maintenance of AF in OSA patients.
Treatment for Atrial Fibrillation in the Presence of Obstructive Sleep Apnoea
Continuous Positive Airway Pressure
The gold standard for OSA therapy is CPAP. The positive pressure keeps the pharyngeal area from collapsing and thus helps alleviate the airway obstruction. Shah et al. conducted a study on consecutive 720 AF patients and found that OSA is independently associated with adverse LV remodelling and clinical outcomes, whereas CPAP therapy is associated with a beneficial effect on LV remodelling.63 Hall et al. recently discovered that in patients with heart failure and OSA, 6–8 weeks of CPAP therapy increased hydroxyephedrine retention, indicating improved myocardial sympathetic nerve function. Cardiac efficiency may be improved by CPAP in patients with severe OSA and heart failure.64 Without appropriate CPAP therapy, AF patients with OSA respond poorly to both pharmacological and nonpharmacological therapy (cardioversion or ablation) with high rates of recurrence.65–67 Monahan et al. studied 61 patients treated with antiarrhythmic drugs for AF who were referred for a sleep study.67 Nonresponders to antiarrhythmic drugs were more likely to have severe OSA than mild OSA (52 % versus 23 %). Severe OSA patients were more likely to be nonresponders as well (70 % versus 39 %). In addition to arrhythmias, OSA patients have an increased BP, pulmonary artery pressure, right ventricular volume, left atrial size and LV mass.50 Therapy with CPAP is associated with lower BP, decreased atrial size and ventricular mass, and a lower risk for AF recurrence after AF ablation.
CPAP therapy substantially reduced the incidence of AF, premature ventricular depolarisation and sinus bradycardia in patients with severe OSA.68 This is in agreement with the study of Kanagala et al. that AF recurrence after cardioversion was 82 % in those with untreated OSA compared with 42 % in the CPAP-treated group.69 Fein et al. demonstrated that AF recurrence following AF ablation in CPAP nonuser patients was significantly higher (HR 2.4) and CPAP therapy resulted in greater AF-free survival rate (71.9 % versus 36.7 %).66 While catheter ablation remains the mainstay therapy for drug-refractory AF, screening AF patients for OSA and initiating CPAP therapy before catheter ablation is advisable.
Emerging Therapy—Autonomic Neuromodulation
Although renal nerve denervation (RND) failed to deliver its promise in controlling drug-refractory hypertension, several preliminary studies indicated that RND may be a promising new therapy for arrhythmias related to hyperactivity of the sympathetic nerves. RND is likely to influence cardiac electrophysiology through alleviating the hyperactive state of the sympathetic nervous system. In fact, recent experimental studies have indicated that RDN was effective in suppressing OSAinduced AF and atrial ERP shortening and could inhibit post-apnoeic BP elevation in an animal model of OSA.49,59 It is noteworthy that Witkowski et al. studied 10 patients with refractory hypertension and OSA who underwent RDN and completed 3- and 6-month follow-up evaluations. They found a decrease in AHI at 6 months after RDN (16.3 versus 4.5), suggesting that RDN may be used to treat hypertension in OSA patients but may also serve as an adjunct therapy to treat OSA.70 A recent study from our group demonstrated that in a rabbit model of OSA, ERP shortening and AF duration induced by OSA can be suppressed by low-level vagal stimulation at voltages not slowing the sinus rate or AV conduction.71 This finding implies that low-level vagal stimulation may be used to treat AF induced by OSA. Inferences from these experimental and clinical studies must be cautiously extrapolated to clinical practice. However, neuromodulation may serve as an adjunct therapy in treating AF in OSA patients by directly modulating the hyperactivity of the autonomic nervous system that facilitates the initiation and maintenance of AF.
Conclusion
OSA is an important but overlooked risk factor for AF. OSA and AF share many common risk factors; therefore, the presence of one may promote the development of the other. OSA also negatively affects the efficacy of pharmacological and ablative therapy for AF. All AF patients should be screened for OSA and therapy to alleviate OSA should be initiated as soon as it is diagnosed in patients with AF.