Thromboembolic stroke and systemic embolism are generally agreed to be the major morbidity/mortality concerns for patients with AF. However, the risk of thromboembolism (TE) is not the same for all AF patients. While ECG rhythm strips of patients with AF are generally indistinguishable, it has long been known that AF in younger patients without co-morbid factors (“lone AF”) carries an extremely low risk for TE, whereas AF in older patients in the presence of specific comorbidities carries a high TE risk.1,2 Thus, AF alone cannot sufficiently explain the risk. However, it has also been long known that older patients with conditions such as hypertension, diabetes and atherosclerotic disease also have an important risk for stroke, even in the absence of AF,3,4 but that these same conditions in the presence of AF have been associated with a two to seven times greater risk of stroke than when AF is absent.5 Thus, the comorbidities themselves also do not fully explain the total risk. Consequently, both AF and comorbidities must interact synergistically to magnify the risk for TE. But, is the synergism dichotomous – AF present or absent, comorbid disorder present or absent – or does synergism have magnitude, depending on the number and severity of the associated disorders and the amount of time one is in AF (AF burden, AFB)? I believe the latter is the case and that clinical trials addressing this point are warranted. Moreover, left atrial appendage (LAA) anatomy and the risk for stasis therein may also be a contributory factor to cardioembolic risk in AF. Thus, the line from AF to stroke is far from straight.
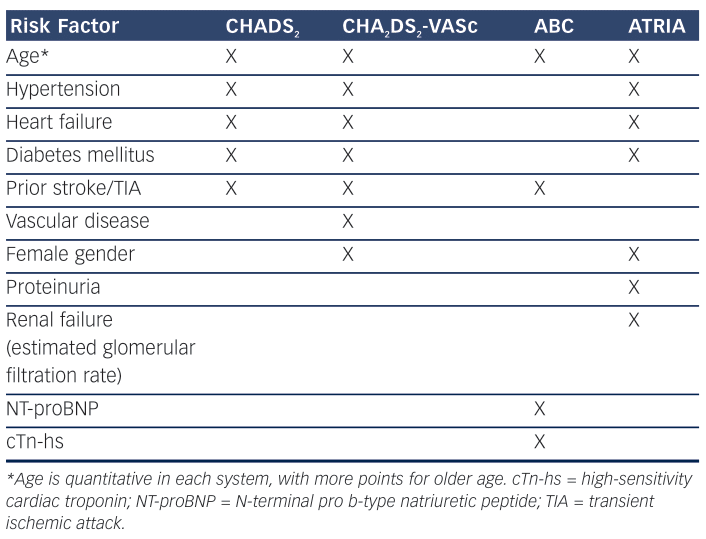
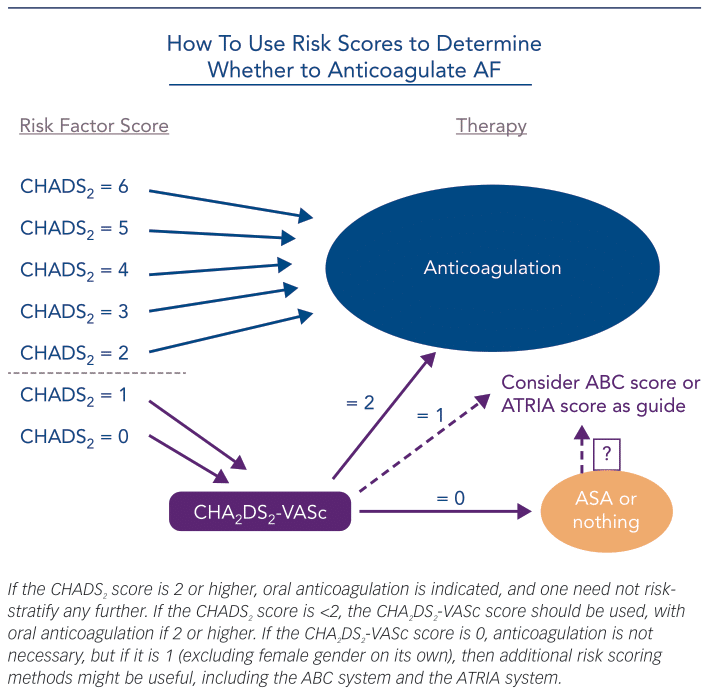
To best understand the risk for TE in patients with AF, both synergism and the magnitude of the underlying components must be recognised. Historically, based on the presence of specific comorbidities, we have determined the risk for TE in AF patients as being either high enough to warrant prophylactic chronic oral anticoagulation (OAC) or too low to justify the risk of OAC-associated bleeding. A decade or so ago, such risk was assessed by determining the CHADS2 score (Table 1), congestive heart failure, a history of hypertension, age 75 years or higher, diabetes (each 1 point), or prior thromboembolic event, e.g. ischemic stroke (2 points), and the paradigm was to determine from the score calculated which AF patients were at high enough risk to warrant OAC – generally agreed to be a score of 2 or more.2 More recently, as we have recognised that stroke is much more likely to be fatal or debilitating than is bleeding, and as we have developed newer oral anticoagulants with preferable efficacy and safety profiles as compared to warfarin, the paradigm has shifted. We now ask which AF patients are at too low a score to warrant avoidance of OAC, with the rest having OAC indicated, and current guidelines1,2 recommend the CHA2DS2-VASc score (Table 1), congestive heart failure, history of hypertension, age 65–74 years (each 1 point) or 75 years and above (2 points), vascular disease and female gender (each 1 point) to make this determination. A score of 0 or 1 being low; OAC being generally recommended for a score of 1 or above (excluding female gender alone).1 Importantly, neither CHADS2 nor CHA2DS2-VASc require symptoms of AF to be present; they are based on readily ascertainable clinical history, demographics and routinely assessed measurements (blood pressure, blood sugar), and they are easy to calculate.
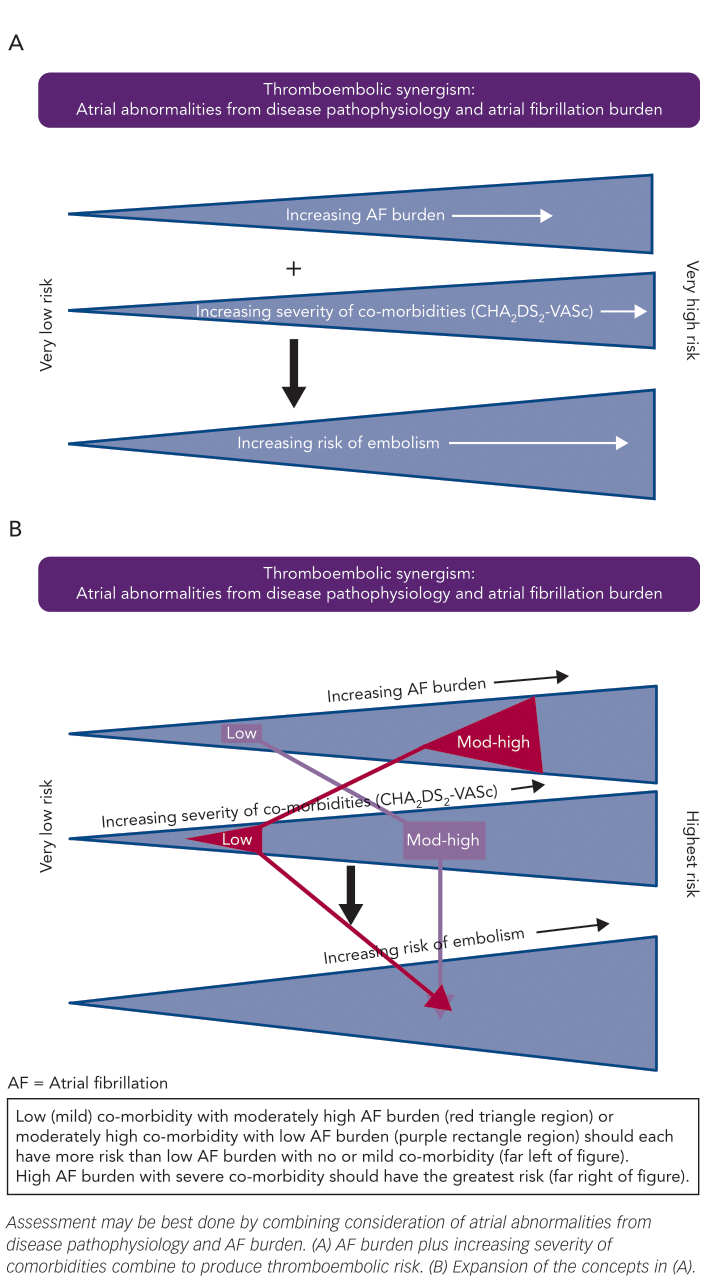
With this move towards offering prophylactic OAC to more AF patients, we must ask ourselves if our current approach is sufficient or if it can be improved still further. I believe it can. First, by recognising that additional considerations might be applied to the AF patients with a CHA2DS2-VASc score of 0 or 1 – as we know that even these patients have some risk of TE – and second, by recognising that we may further improve our selectivity if we utilise an understanding of the magnitude synergism that exists between AF and comorbidities in generating TE risk (Figure 1) and laboratory values that may reflect it. That such an interplay exists is not entirely a new concept. We have known for years that the risk of stroke with AF increases with age6 – one of the factors in both the CHADS2 and CHA2DS2-VASc scoring systems. Moreover, that such an interplay should also hold true for other important comorbidities is a concept that I detailed in an editorial in 2016.7
AF Burden
Several recent sources of evidence suggest that AF burden has importance in determining the likelihood of a thromboembolic event. Botto and colleagues,8 for example, assessed the interaction between AFB and CHADS2 factors with respect to risk for embolic stroke. They assessed AFB in three groups: no AF, AF>5 min and AF>24 h. There were no substantial differences in the frequency of each AFB duration group within any of the CHADS2 component groups. The rate of TE events increased linearly with the presence and duration of AF, so too as the CHADS2 score increased from 0 to 1 to 2. Notably, the patients with a CHADS2 score of 0 were at low risk, even if they had long-lasting AF, as were patients with a CHADS2 score of 1 if AFB was >5 min but <24 h, and patients with a CHADS2 score of 2 if they had no AF. By contrast, patients with a CHADS2 score of 3 or higher demonstrated high risk, even without AF being recorded, as did patients with a CHADS2 score of 2 if they had AF >5 min. Similar (though not identical) to this were the observations of Van Gelder et al. who reported events with implanted device-detected AF in a population with a mean CHADS2 score of 2.3 using the groups: no subclinical AF (no AF or AF<6 min), AF of 6 min to 6 h, AF of 6–24 h and AF >24 h.9 The stroke rates in the first three groups were not significantly different, with hazard ratios of 1, 0.93 and 1.39, respectively, whereas the AF >24 h group had a hazard ratio of 3.86 compared with the no subclinical AF group. Likewise, Boriani and Pettorelli reported that although a device-detected maximum daily burden of AF of at least 5–6 min was associated with an increase in the risk of stroke, the risk was particularly increased if the daily AFB was at least 1 h.10
Consistent with the above, using 14-day continuous ambulatory recordings to document AF of at least 30 s, Go et al. demonstrated that during follow up the rate of validated thromboembolic events off anticoagulants was 2.52/100 patient-years, with a higher crude rate with greater AF burden.11 After adjustment for ATRIA stroke risk score (see below), there was a 9 % increase in the odds of TE per 10 % increase in AFB, which was borderline significant. Likewise, Ganesan et al. reported that morbidity and mortality with AF were both higher in patients with non-paroxysmal AF than in patients with paroxysmal AF, but with no differences in bleeding rates.12 If the increased adverse events were due to more advanced comorbidities in the non-paroxysmal patients, then it would be expected that they would also have higher bleeding rates – which they did not. Thus, the mere presence versus absence of AF is not enough of a consideration, especially in those with lower CHA2DS2-VASc scores. Here, a score of 1 plus a high AFB probably justifies OAC, whereas a similar score with only brief, infrequent AF may not. Unfortunately, we are not yet sophisticated enough to accurately determine what threshold of AFB is important at any specific comorbidity magnitude, although we can understand the concept of the interaction (Figure 1). Lower AFB may be enough to trigger clot formation when the number and severity of comorbidities (and hence their effect on the atria) is high, whereas a greater AFB, such as 24 h or more, may be necessary with a lower CHA2DS2-VASc score.
Magnitude of Comorbidities
Determining how the number and degree of comorbidities is related to TE risk is not as easy as it is for AFB. Certainly, the CHA2DS2-VASc score relates well to the number of contributory comorbidities, but only age is considered in any quantitative way. Yet, if one considers pathophysiologically how disease can contribute to thrombus formation in the left atrium, the process cannot simply be “all or none”. Multiple factors interplay in this process, including both stasis of blood from left atrial anatomical alterations and mechanical dysfunction, as well as abnormal coagulation factors – many of which relate to abnormal endothelial function. Mechanical dysfunction can result from structural remodelling with histopathological alterations in the diseased atrial wall, such as fibrosis, infiltration or inflammation, with the first of these occurring commonly and progressively with age and with disorders that increase LA pressure and volume, resulting in an atrial cardiomyopathy.13–15 A tachycardic myopathic component from AF itself is then an additive contributor. Numerous abnormal coagulation factors have been described in both diseased and fibrillating atria, as well as systemically in AF patients. They include an increased ratio of procoagulant factors versus normal anticoagulant factors, only a few of which are affected by antiplatelet agents. The latter may explain why aspirin is not comparable to OAC in the prevention of TE in at-risk patients with AF. More specifically, reports of coagulation alterations during AF include increases in: factor VII, fibrinogen, D-dimer, prothrombin fragment 1.2, thrombin–antithrombin complexes, thrombin generation, plasminogen activator inhibitor-1, von Willebrand factor, P-selectin, β-thromboglobulin, platelet factor 4, soluble CD40 ligand and superoxides in the left atrial appendage (which degrade nitrous oxide).16–24
Logically, more numerous or advanced comorbidities will have a greater impact on the structural and electrophysiological remodelling that enhances the frequency and duration of AF as well as on the severity of endothelial dysfunction and abnormal coagulation factors. That this is likely the case can be gleaned from the Framingham risk score data and its relationship to 5-year stroke risk. In 2003, Wang and colleagues reported on the risk of stroke or death versus the height of systolic blood pressure and the presence and number of specific additional factors, including diabetes, smoking, prior MI and left ventricle (LV) hypertrophy by ECG, stratified by age.25 There was a steady and age-related increase in the risk of stroke or death as the systolic blood pressure rose from 130 to 170 and as the number of comorbidities increased – from approximately 10 % to over 50 % in 60 year olds and from approximately 25 % to over 70 % in 70 year olds. Similarly, the yearly risk of stroke has been shown to increase in AF patients as the degree of systolic LV dysfunction increases by echocardiography,26–28 and the yearly risk of major cardiovascular and cerebrovascular events has been shown to increase progressively as the degree of left atrial fibrosis (demonstrated by left atrial gadolinium enhancement severity) increases.29 These are quantitative rather than present/absent determinants. Finally, with respect to endothelial dysfunction and its enhanced propensity for thrombogenesis, Lim et al. showed that there is a “significant stepwise increase in endothelial dysfunction measured by asymmetric-dimethylarginine from controls to lone AF to AF with comorbidities (p<0.001)”.30
Importantly, many of these structural and coagulation changes do not normalise with the restoration of sinus rhythm by drug therapy or by ablation. Rhythm normality does not necessarily mean functional or rheological normality. Both the AFFIRM and RACE trials reported more strokes in patients in their rhythm control arms than in their rate control arms.31,32 This is considered to have resulted from higher rates of OAC reduction or discontinuation upon presumption of maintained sinus rhythm by the patients’ physicians, and unrecognised (subclinical) recurrences of paroxysmal AF in these patients. However, incomplete reverse remodelling must also be considered. Using magnetocardiography, Lehto et al. demonstrated that magnetocardiographically detected atrial electrophysiological alterations in persistent AF diminish during maintained sinus rhythm after cardioversion, but only incompletely.33 Lo and Chen reported only partial reversal of structural remodelling after return to sinus rhythm.34 In their study of mitral stenosis patients with AF pre-existing their cardioversion and commissurotomy, Fan and colleagues reported the recovery course of electrical remodelling to be prolonged and heterogeneous in AF patients, and that regional conduction abnormalities were irreversible.35 In patients who underwent ablation for their AF, Masuda et al. reported that increased left atrium (LA) ablation lesions, better LA ejection fraction and paroxysmal AF were associated with worsening of LA function post-ablation as compared to pre-ablation, rather than improvement.36 Using MRI and magnetic resonance spectroscopy, Wijesurendra et al. reported that LA function did not normalise post-ablation, regardless of both recovery of sinus rhythm and freedom from AF.37 Finally, Kusa et al. noted that LA appendage flow velocity remained low in 22 % of their post-ablation patients, even after 6 months of sinus rhythm, and that a CHA2DS2-VASc score of 2 or more was an independent predictor of low LA appendage flow velocity.38 These observations support the consideration of AF synchronously with any associated structural abnormalities when considering OAC, and that the latter may not only persist but may increase with some forms of rhythm control. They also support the current recommendation that OAC generally be continued in AF patients, even if sinus rhythm is restored with an antiarrhythmic drug or ablation, if the CHA2DS2-VASc score would warrant it and if AF was still present.
Biomarkers
In addition to the above considerations, there is growing evidence that the presence of abnormal biomarker activity may also correlate with the risk for TE in patients with AF. Although one might assume that biomarker abnormalities would simply reflect the degree of comorbidity severity, such as LV dysfunction, hypertrophy, inflammation, fibrosis and the like,39–42 and therefore would have to be increased in patients with higher CHA2DS2-VASc scores and higher stroke risk, it is of interest that in the dabigatran versus warfarin AF trial where this was examined, troponin I and NT-proBNP levels were additive to the CHADS2 score in correlating with stroke and systemic embolism, pulmonary embolism, myocardial infarction and non-hemorrhagic vascular death.40 The biomarkers raised the c-statistic for these endpoints from 0.68 to 0.72 (p<0.0001). At a minimum, as greater biomarker abnormalities may correlate with comorbidity severity, they may be one way to reflect comorbidity magnitude and it may be appropriate to consider OAC in patients with a low CHA2DS2-VASc score but in whom such biomarkers are abnormal – especially if in the setting of a comorbidity factor aside from age and gender. At least a prospective interventional trial to this point is worth considering.
Moreover, an alternative risk score method utilising biomarkers has already been proposed and evaluated. In 2016, Oldgren, Hijazi and colleagues reported on the development and validation of a biomarker-based stroke risk score: the ABC score.43,44 This score includes age, biomarkers (N-terminal fragment B-type natriuretic peptide [BNP] and high-sensitivity cardiac troponin) and clinical history (prior stroke)
(Table 1) and is calculated from a nomogram using a sliding scale for each of the components. The ABC score achieved slightly higher c-indices than CHA2DS2-VASc. Nonetheless, this scoring system has not yet been widely used or recommended in major guidelines – in part, perhaps, because the biomarker values are not routinely assessed in most clinical care settings and because it requires use of nomogram for its determination rather than a simple, quick calculation using easy to recall point values of 1 or 2. However, because currently used risk
scoring systems are practical but still somewhat limited in their stroke prediction accuracy, biomarkers might improve our assessments. In addition to BNP or pro-BNP and troponin measurements, D-dimer, inflammatory growth differentiation factor-15, micro-RNAs, galectin-3 (which correlates with myocardial fibrosis), C-reactive protein (inflammation) and determinants that reflect renal dysfunction (creatinine, cystatin C) have also been considered for use.
With respect to considering renal status in risk assessment, higher risk for TE in AF patients has been demonstrated to be inversely related to estimated glomerular filtration rate.45 Accordingly, Singer et al. have reported on an additional approach, the ATRIA score,46 as an alternative with a greater C-index than they found with CHADS2 or CHA2DS2-VASc. The ATRIA score (Table 1) is based on age, female gender, diabetes mellitus, heart failure, hypertension, proteinuria and estimated glomerular filtration rate stratified by the presence or absence of prior stroke. Without a prior stroke, points are assigned as: 6 for age 85 years and above, 5 for age 75–84 years, 3 for age 65–74 years, 0 for age <65 years, and 1 each for female gender, diabetes, congestive heart failure, hypertension, proteinuria and an estimated glomerular filtration rate <45cc/min or end-stage renal disease. With a prior stroke, the points are the same except: 9 for age 85 years and above, 7 for age 75–84 years, 7 for age 65–74 years and 8 for age <65 years. A score of 0–5 points is considered low risk, 6 points is considered risk and 7–15 points is considered high risk. Thus, simplistically, it adds renal dysfunction to the components used in the CHADS2 system. As with the ABC score approach, the ATRIA scoring method has not yet been widely adapted. Moreover, its relative performance against CHADS2 or CHA2DS2-VASc appears to depend on population specifics, such as prior stroke rates.47 Nonetheless, I think both systems may be appropriate to consider in patients in whom CHA2DS2-VASc itself does not suggest a certain indication for OAC but concern may exist (Figure 2). Consider, for example, a 62-year-old man with hypertension associated with LV hypertrophy and diastolic dysfunction but no other CHA2DS2-VASc factors, or a 42-year-old diabetic man with moderate renal insufficiency but no other CHA2DS2-VASc
factors. If each had a high AFB, I would likely start OAC, but if AFB were low, consideration of the ABC score or ATRIA score may be quite helpful in the decision.
Left Atrial Appendage Anatomy and Function
Coupled with considerations of the magnitude of comorbidities, there is a growing set of data to suggest that left atrial appendage anatomy may be an additional factor to consider in determining TE risk in patients with AF. However, the data is somewhat conflicting and its role in assessing whether or not to anticoagulate a patient with a low CHA2DS2-VASc score is not yet clear. For example, Di Biase et al. proposed a LA appendage anatomic pattern scheme to stratify risk for TE in AF patients.48 Four major patterns were described: cactus, windsock, chicken wing and cauliflower. Patients with chicken wing morphologies were felt to be at lower risk for stroke than those with other patterns, especially cauliflower. Concordantly, Petersen et al. reported that non-chicken wing morphologies were associated with lower LAA emptying velocity and higher prevalence of spontaneous echo contrast than chicken wing morphology, irrespective of the underlying type of AF.49 Relatedly, over a decade ago Goldman et al. demonstrated that the annualised rate of cardioembolic events in AF increased as peak LAA flow velocity decreased.50 However, somewhat in contrast, Anselmino et al. reported that windsock, cauliflower and chicken wing morphologies were independently related to the risk of silent cerebral ischemia by imaging.51 The descriptive shape of the LAA may not be the only LAA feature that plays a role in TE risk. In a two-case report series, Kreidieh and Valderrabano suggested that an elongated, narrow LA appendage that tapered slowly into a pointed tip – “a rare variant of the commonly classified chicken wing morphology” – had a specifically notable risk for TE.52 Khurram et al. reported that smaller LA orifice diameters and more LAA trabeculation independently correlated with higher stroke risk.53 Yamamoto et al. reported that the number of lobes, rather than the descriptive pattern, may be the determinant of LAA TE risk.54 In a prospectively enrolled population of 633 consecutive patients (of whom 594 had evaluable transesophageal echocardiograms), multivariate analysis revealed CHADS2 score (p=0.002), LV ejection fraction (p=0.01), degree of spontaneous echo contrast (p=0.02), LA volume (p=0.02) and number of LAA lobes (p<0.001) to be independently associated with LAA thrombus. Most patients with LAA thrombus (94.4 %) had three or more lobes, whereas thrombus was observed in only 2 of 296 patients (0.7 %) with 1 or 2 lobes. In an analogous manner, Korhonen et al.55 assessed LA appendage morphology by cardiac CT in 111 patients with suspected cardiogenic stroke without known AF. The distribution of morphology types differed significantly between the matched stroke and control groups. Cactus and windsock were less common in the stroke patients than in the controls, whereas chicken wing and especially cauliflower were more common in the stroke patients. Similarly, increased LA appendage volumes were larger in the stroke patients, and single-lobed appendages were overrepresented in the stroke patients. Thus, the number and size of the lobes and their orifices may be more important contributory features than
the shape the lobes take. While the consideration of LA appendage anatomy and risk for TE clearly needs further investigation and clarification, it seems likely that any alteration in which emptying is impaired, flow is impaired and stasis is increased should relate to risk for clot formation within the appendage. However, as LA appendage anatomical patterns require imaging to ascertain, we are not yet ready to add them to our risk stratification consideration for patients with a low CHA2DS2-VASc score.
Conclusion
We have come a long way in understanding: (1) those factors that promote the risk of TE in patients with AF, and (2) that we can utilise this understanding in a way to determine when the risk of TE is high enough to warrant prophylactic OAC – most of the time. However, the value of quantitative mechanistic synergism and utilising AF burden, laboratory means and imaging means to quantify associated tissue and coagulation pathophysiology so as to improve selectivity in our decision has not yet been adequately appreciated. Clinical trials to test the importance and validity of these considerations are necessary. Thus, we still have a road on which we must continue travelling. Or, as a fortune cookie I was recently served for dessert said: “thromboembolic risk – there is still more to know.”
Clinical Perspective
- Risk for thromboembolism in AF is determined by both the amount of AF and the presence and magnitude of contributing comorbidities.
- Current risk-scoring methods do not adequately consider the magnitude synergism of the AF and comorbidity interaction.
- Current risk-scoring approaches can be used in combination to better determine who is at lowest risk, not requiring anticoagulation.
- Left atrial appendage morphology may also be considered in risk-assessment.