AF remains the most prevalent cardiac arrhythmia and the most prominent cardiac aetiology for stroke.1 The prevalence of AF continues to grow globally, and it is estimated that by 2050, AF will be nearly three times more common than in 2023.1 The increased prevalence of AF is propagated by the increasing age of the general population worldwide, combined with an elevated presence of risk factors, such as diabetes, hypertension and obesity.2
Device-detected AF, otherwise known as atrial high-rate episodes (AHREs), is an emerging clinical entity, defined as episodes of asymptomatic atrial tachyarrhythmias with an atrial rate ≥175 BPM occurring for at least 5 minutes.3 AHREs are commonly seen in individuals without a diagnosis of AF and can only be detected through cardiac implantable electronic devices (CIEDs) (such as ICDs, CRT devices and permanent pacemakers [PPMs]) due to properties of continuous cardiac monitoring and storage of data).3–6 While AHREs are heavily considered a predecessor for AF, as evidenced by an estimated three to five times increased risk of developing clinical AF, they are distinct from AF based on how they are documented.3,4,6 Clinical AF is diagnosed by surface electrocardiography, whereas AHREs can only be detected through CIEDs.4,7 Although AHREs affect asymptomatic individuals, these individuals may remain at an increased risk for ischaemic stroke, mortality and adverse cardiac events.4,8 While guidelines recommend therapeutic anticoagulation or left atrial appendage occlusion in patients with AF, there remain limited guidelines for stroke prevention with AHREs, leaving a significant population of individuals at risk of inadequate care.4
Device-detected AF has emerged as an issue of concern in individuals with CIEDs. Previous systematic reviews and meta-analyses on the topic have had many limitations, one of which was their need to evaluate outcomes in individuals with no prior history of AF, who experienced device-detected AF. Therefore, we conducted a meta-analysis to evaluate the risks of new-onset clinical AF, thromboembolism and all-cause mortality in individuals with no prior history of AF who experienced AHREs.
Methods
This study was performed in line with PRISMA and AMSTAR guidelines.9,10 Systematic methods were implemented to search for relevant studies, assess study eligibility and evaluate study quality.
Eligibility Criteria
Only comparative studies published in the English language involving research on individuals with CIEDs who experienced device-detected AF were considered. Grey literature was not considered part of the eligibility criteria given that only published studies were eligible for evaluation.
Inclusion and Exclusion Criteria
Studies were included if they enrolled individuals with device-detected AF detected by CIEDs, such as ICD, CRT and PPM devices. There was no restriction in inclusion criteria based on duration of AHREs, nor was there restriction in inclusion criteria based on duration of outcomes assessed. Given that AF has already been established as a significant independent risk factor for stroke and mortality, a prior history of AF was an exclusion criterion. Furthermore, systematic reviews, meta-analyses, case reports, case series and cross-sectional studies were excluded as they lacked a control group for comparison.
Literature Search and Screening
A comprehensive literature was conducted using the PubMed, EMBASE, ClinicalTrials.gov and Cochrane databases for individuals with CIEDs who experienced device-detected AF from the inception of each database to March 2024. The following search terms/keywords were used both individually and in combination: “atrial high-rate episodes”, “atrial fibrillation”, “subclinical atrial fibrillation”, “stroke”, “thromboembolism”, “mortality” and “cardiac implantable electronic devices”.
Furthermore, the reference lists of the included studies were manually evaluated to identify relevant articles. There were no restrictions on the search regarding publication year or sample size. A two-step screening process was conducted by two evaluators for title and abstract screening, as well as full-text screening. Studies were considered ineligible at the full-text screening stage based on pre-specified eligibility requirements. Thorough discussions were held regarding disagreements that arose among the reviewers until agreements were reached.
Data Extraction
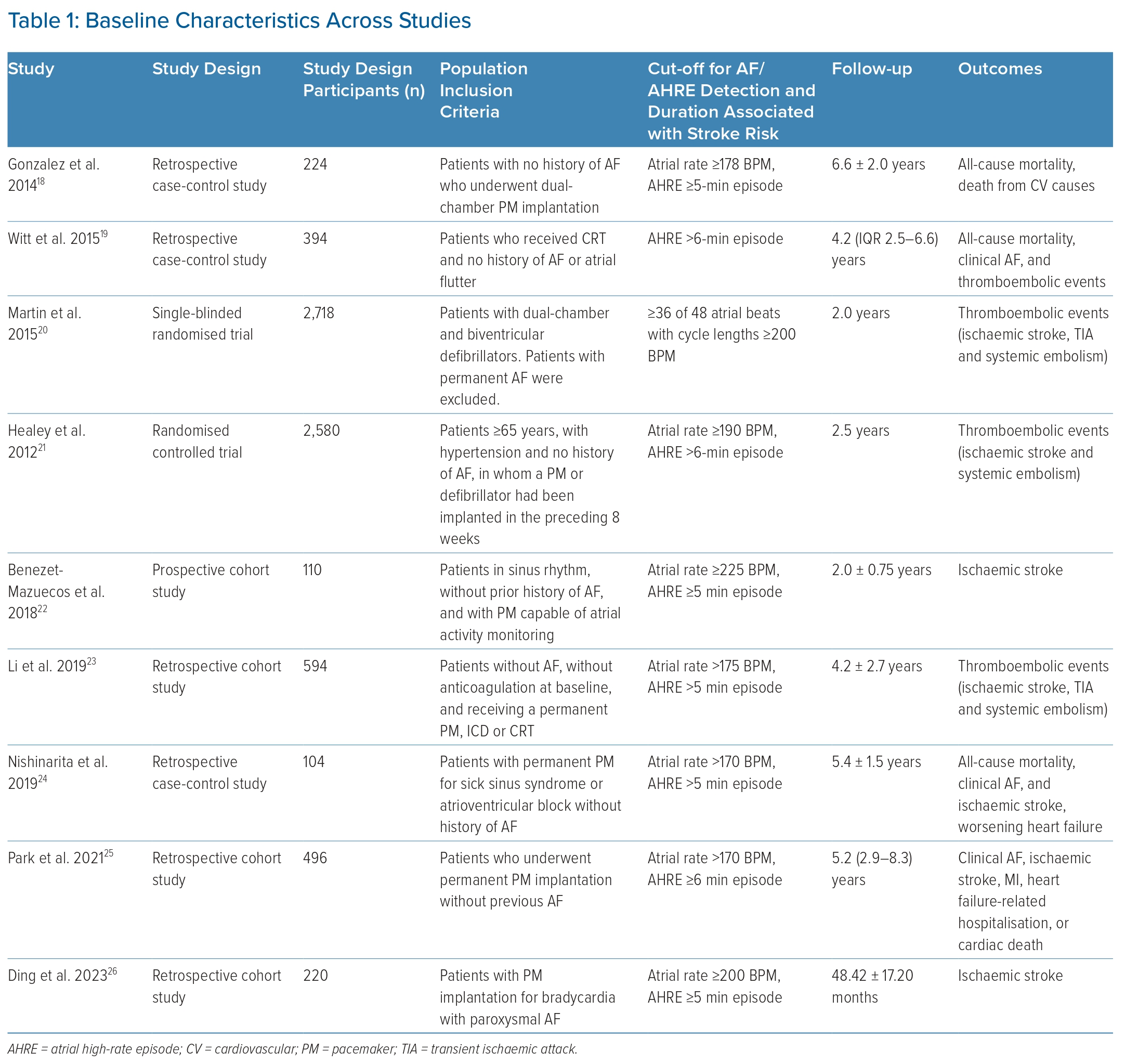
Using a standardised data extraction form, records obtained from the electronic databases were extracted in a duplicate manner by two independent reviewers. Data extracted included study characteristics, baseline patient characteristics and outcomes (new-onset clinical AF, thromboembolism and all-cause mortality). Definitions of clinical AF, outcomes, and threshold values for the detection and duration of device-detected AF were determined by the individual studies. Possible confounding factors such as CHA2DS2-VASc score and individual cardiovascular profiles were also noted.11
Risk of Bias and Quality Assessment in Individual Studies and Across Studies
The same investigators both systematically and independently assessed the methodological quality of observational studies using the Newcastle-Ottawa Scale and evaluated randomised controlled trials (RCTs) using the Risk of Bias Assessment Tool from the Cochrane Handbook for RCTs.11,12
Supplementary Table 1 presents the risk of bias assessment of the included studies. Publication bias was by visual inspection of funnel plots when data were available from a minimum of at least three studies. Visual inspection of the funnel plots revealed no evidence of publication, reflecting low heterogeneity overall (Supplementary Figures 1–3).
Outcomes
Outcomes of interest included clinical AF, thromboembolic events and all-cause mortality.
Data Synthesis
We performed a meta-analysis for each outcome by using the RR as a key measure of effect. Content from data extraction included relevant information applicable to each study such as the quantity of events in both exposed and unexposed groups, total sample size, and CI for the RR estimates. The RR, indicating the ratio of the outcome risk in the exposed compared to the unexposed group, was calculated for each study. We then used the Mantel-Haenszel method, a fixed-effects model, to pool the relative risks of each study to obtain an overall assessment of the RR for each outcome studied.13 The rationale for pooling RR using the Mantel-Haenszel method was based on its suitability for dichotomous data while controlling for confounding factors.13 Through the Mantel-Haenszel method, we were able to calculate a weight-based summary encompassing the RR with an associated 95% CI for each outcome studied.13 Given that prior data have shown AHRE duration ≥24 hours to be associated with an increased risk of thromboembolic events, we conducted a subgroup analysis of the included studies to evaluate the risk of thromboembolism based on the duration of AHRE episodes, comparing AHRE durations <24 hours to AHRE durations ≥24 hours. Only those studies which provided thromboembolic event data for both AHRE durations <24 and ≥24 hours were identified and included in our subgroup analysis. Two-tailored p-values were calculated with a significance threshold of 5% used to determine significance. Heterogeneity among the included studies was evaluated based on the Cochran Q statistics and I2 statistics.14,15 An I2 >30% was considered to have moderate heterogeneity, >75% was considered to have high heterogeneity and a p-value <0.1 was used to determine the significance of heterogeneity.15,16 All analyses were performed and calculated using RStudio version 2023.12.0+369.17
Results
Study Selection
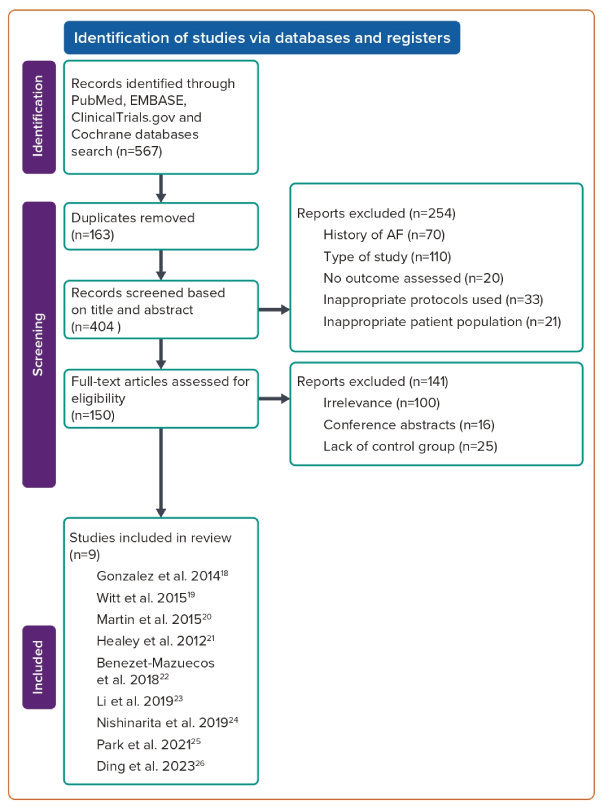
A total of 567 studies were initially identified through the literature search. After removing duplicate studies, 404 studies were eligible for the title and abstract screening process. During the title and abstract screening process, 254 studies were disqualified based on study type, protocols used, wrong patient population and lack of study outcomes. Furthermore, records that involved patients with a prior history of AF were also disqualified. The remaining 150 studies were subjected to full-text screening. Ultimately, a total of nine studies met inclusion criteria with a combined enrolment of 7,440 patients who had AHREs without any prior history of AF at the time of baseline evaluation (Table 1 illustrates the baseline characteristics across studies).18–26 Figure 1 presents a PRISMA flow diagram of the study selection process.
AHREs and Clinical Atrial Fibrillation
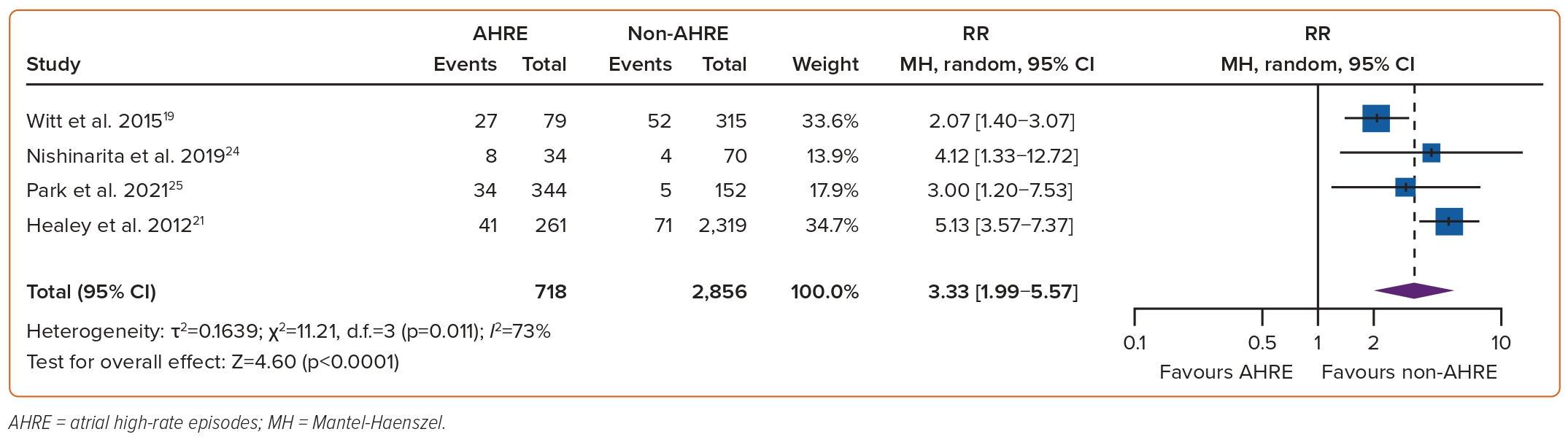
The association between AHREs and the risk of clinical AF was investigated in four studies, which included a total of 3,574 individuals.19,21,24,25 The RR of developing clinical AF among patients with AHREs, compared to those without AHREs, was found to be significantly associated at 3.33 (95% CI [1.99–5.57]; p<0.0001, Figure 2). Funnel plots showed a symmetrical distribution of studies with no evidence of publication bias (Supplementary Figure 1).
AHREs and Thromboembolism
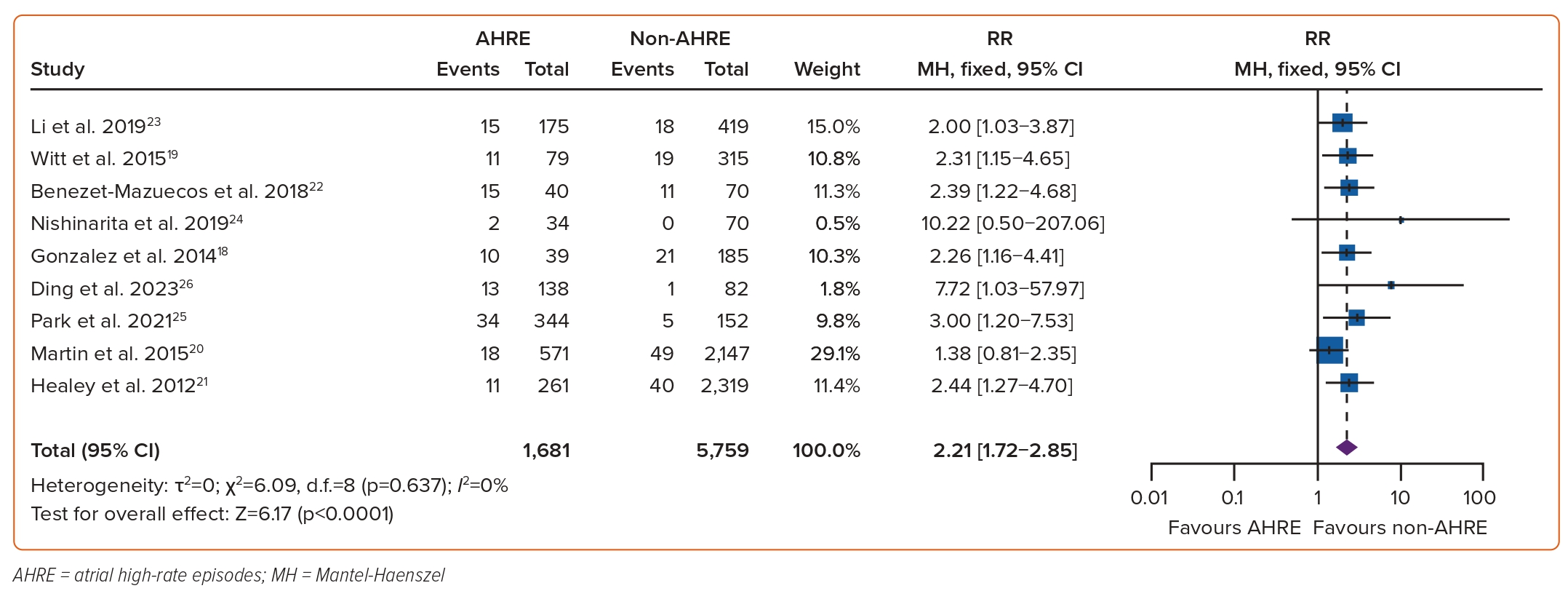
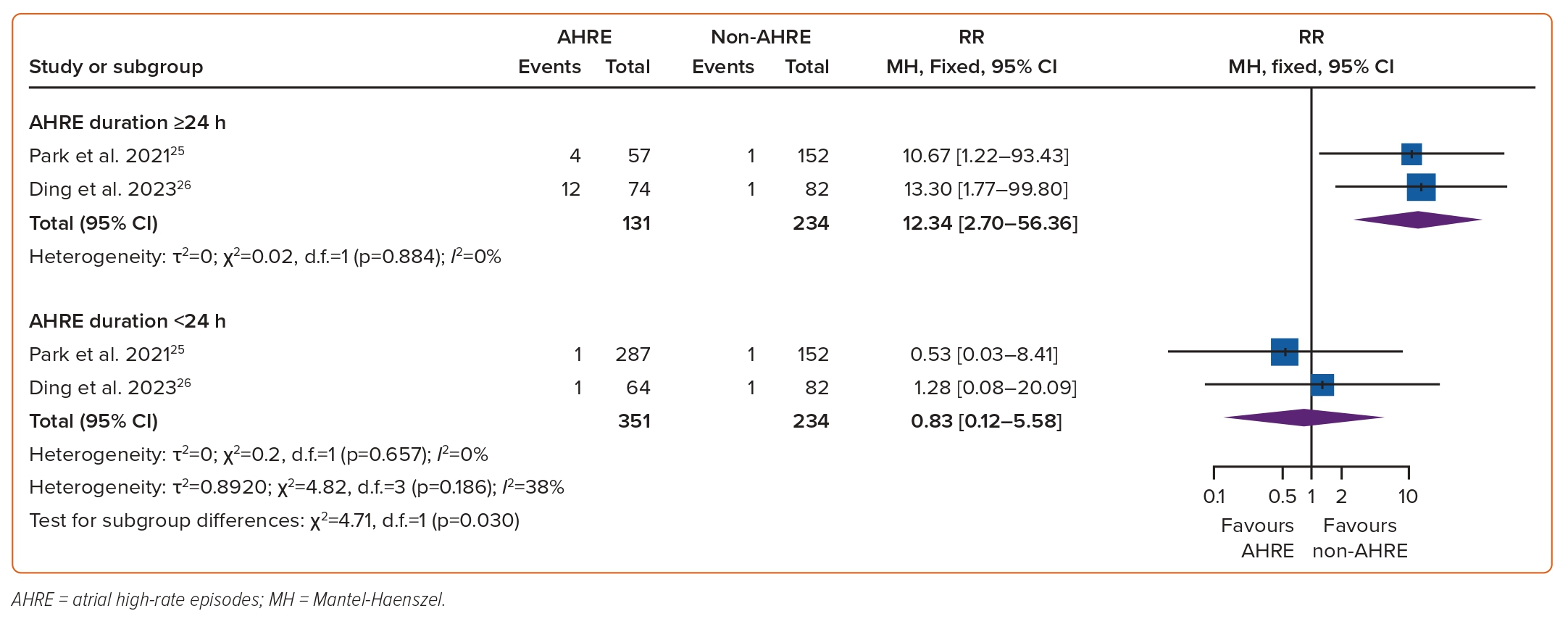
The association between AHREs and the risk of thromboembolism was investigated in nine studies which included a total of 7,440 individuals.18–26 The RR of developing thromboembolic events among patients with AHREs, compared to those without AHREs, was found to be significant at 2.21 (95% CI [1.72–2.85]; p<0.0001, Figure 3). Funnel plots showed a symmetrical distribution of studies with no evidence of publication bias (Supplementary Figure 2). A subgroup analysis based on the duration of AHRE episodes found that among episodes <24 hours, the risk of thromboembolism was insignificant (RR 0.83; 95% CI [0.12–5.58]). However, there was a significant risk of thromboembolism among AHRE episodes ≥24 hours, compared to those without AHREs, (RR 12.34; 95% CI [2.70–56.36]; test for subgroup differences: p=0.030, Figure 4.)
AHREs and All-cause Mortality
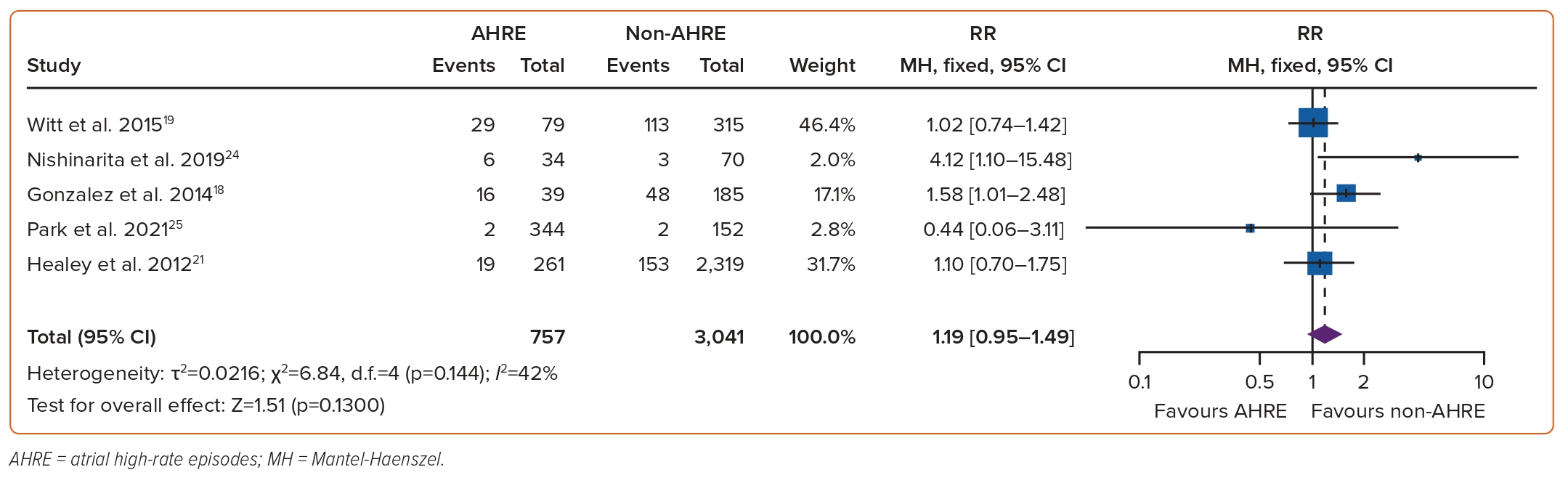
The association between AHREs and the risk of mortality was investigated in five studies, which included a total of 3,798 individuals.18,19,21,24,25 The RR of mortality among patients with AHREs, compared to those without AHREs, was found to be insignificant at 1.19 (95% CI [0.95–1.49]; p=0.13, Figure 5). Funnel plots showed a symmetrical distribution of studies with no evidence of publication bias (Supplementary Figure 3).
Discussion
AHREs and Clinical Atrial Fibrillation
Our finding of an increased risk of developing clinical AF in patients with AHRE, without prior AF, aligns with previous individual studies as evident in the ASSERT trial, which found that the detection of subclinical atrial tachyarrhythmias was associated with an increased risk of AF (HR 5.56; 95% CI [3.78–8.17]; p<0.01) among patients with a pacemaker who had no prior history of AF.21 Nishinarita et al. noted P wave dispersion, a notable predictor of clinical AF, to be significantly longer among individuals without a prior history of AF who experienced AHREs, compared to those who did not (62.6 ± 13.1 versus 38.2 ± 13.9 ms; p<0.01), with P wave dispersion also being a significant risk factor for AHREs themselves (HR 1.11; 95% CI [1.06–1.17]; p<0.01).27
The remodelling of atrial electrical activity and structure by the diseases that warranted the need for CIEDs implantation likely contributed to the elevated risk of developing AHREs and ultimately the increased risk for AF due to disturbances in cardiac conduction prior to CIED implantation.27 This pathophysiological explanation is supported by the fact that electrophysiological abnormalities encompassing the sinus node have been shown to trigger and propagate AF.27 Furthermore, the increased risk of developing clinical AF can be attributed to amplified processes of atrial dilation, fibrosis, inflammation and oxidative stress occurring in patients with AHREs that result in AF.28 Biomarkers noted to reflect such processes, such as tissue inhibitors of metalloproteinases, interleukin-6, NT-proBNP, serum amyloid protein A, and NT-proANP, have been reported to be significantly associated in those who experienced AHREs in comparison to those who did not.28
AHREs and Thromboembolism
Our meta-analysis noted a significant association between AHREs, among individuals without a prior history of AF, and the risk of thromboembolism. This conflicts with a prior study by Chu et al., who noted new-onset stroke or systemic embolism were insignificant outcomes (p<0.395) in individuals with subclinical AF detected compared to those without subclinical AF.29 In contrast, the ASSERT trial found an increased risk of systemic embolism or ischaemic stroke in individuals without a history of AF who experienced AHREs in comparison to those who did not (HR 1.77; 95% CI [1.01–3.10]; p=0.047).21,30 The discrepancy in thromboembolic risk is likely to be due to the variation in the duration of AHREs, as reflected in our subgroup analysis which found an increased risk of thromboembolism in AHREs ≥24 hours. This is highlighted in a study by Capucci et al., who noted AHREs >24 hours were associated with a significantly increased risk of embolic phenomena (adjusted HR 3.1; p=0.04).30,31 Similarly, an analysis of the ASSERT trial by Van Gelder et al. found the risk of stroke to be nearly entirely driven by AHREs >24 hours, as patients with episodes >24 hours had an increased risk of systemic embolism or ischaemic stroke compared to those who did not experience AHRE (p=0.003).32
Although our findings suggest AHREs to be associated with an increased risk for thromboembolism, further studies are warranted to analyse the duration of AHREs which propagate the likelihood of stroke.
Prior studies have explored the relationship between clinical risk factors of stroke and thromboembolic events in AHREs, as seen in a study by Kaplan et al. who found the risk of thromboembolic events in device-detected AF was significantly associated with a rising CHA2DS2-VASc score (p<0.001).33 Increased risk of stroke can be further attributed to the hypercoagulable state that occurs as a result of atrial cardiomyopathy, in which both endothelial and mechanical abnormalities involving the dilated atria amplify the risk of embolic stroke.7 Additional studies are indicated to assess the relationship between stroke risk factors and thromboembolic outcomes in AHREs
AHREs and Mortality
Our study found the association between AHREs and the risk of all-cause mortality to be insignificant, which conflicts with prior studies. In a retrospective study by Jacobsson et al., outcomes concerning mortality were found to be insignificant among those with device-detected AF (p=0.67).34 However, the burden of AHREs themselves was low overall in the study, which may have played a role in the lack of significance noted.33 In contrast, a longitudinal study found increased mortality among individuals without a history of permanent AF who experienced AHREs 48 hours before ventricular arrhythmias, in comparison to those who did not experience AHREs (HR 2.67; 95% CI [1.68–4.23]; p<0.01), with survival differences noted to be significant even after excluding those with any subtype of AF (p<0.01).35 The increased risk of mortality noted in our findings may be attributed to the duration of AHRE, as Park et al. noted patients with a high-burden of device-detected AF (≥24 hours) had a significantly increased risk of cardiac mortality compared to those who had a low burden of device-detected AF (<24 hours) and to those who did not experience AF (p=0.018).25 The increased risk of mortality in device-detected AF ≥24 hours is likely multifactorial, propagated by an increased progression towards clinical AF, resulting in a higher occurrence of adverse events, such as acute heart failure exacerbations and ischaemic strokes.25 The discrepancy in mortality outcomes noted may further be attributed to the variability of cardiovascular risk factors among study subjects, such as dyslipidaemia, vascular disease, advanced age and hypertension along with the severity of cardiac comorbidities that warranted the need for CIEDs implantation, such as ischaemic heart disease/ventricular dysfunction.36
Future Perspectives
AHREs remain a focus of discussion regarding both their prognostic significance and their management. The 2023 American College of Cardiology/American Heart Association/American College of Clinical Pharmacy/Heart Rhythm Society guidelines on the management of AF brought new insight into addressing subclinical AF, suggesting initiating anticoagulation to be reasonable in patients with AHREs lasting at least 24 hours who have a CHA2DS2-VASc score ≥2 or similar stroke risk.37 Furthermore, guidelines consider initiating anticoagulation to be reasonable among those with a CHA2DS2-VASc score ≥3 who experience device-detected AF between 5 and 24 hours while citing no benefit in those who experience device-detected AF <5 minutes in duration.37 These recommendations, however, are categorised as moderate and weak levels of strength respectively, highlighting the continued uncertainty of the utility of anticoagulation in AHRE.37
Recent RCTs have aimed to investigate the role of anticoagulation in AHRE. A meta-analysis of the NOAH-AFNET 6 and the ARTESiA RCTs noted significant decreases in ischaemic stroke (RR 0.68; 95% CI [0.50–0.92]) and a composite of systemic embolism or all-cause stroke (RR 0.65; 95% CI [0.49–0.86]) with oral anticoagulation.38 Interestingly, while there were increased risks of major bleeding (RR 1.62; 95% CI [1.05–2.5]) and a composite encompassing all-cause mortality or major bleeding (RR 1.16; 95% CI [1.00–1.35]) with oral anticoagulation, there was no difference noted in fatal bleeding (RR 0.79; 95% CI [0.37–1.69]).38 Ultimately, the decision to prescribe anticoagulation in patients with AHRE mandates providers to weigh the risk of stroke with the concern of perpetuating major bleeding and mortality. The feasibility of reversing major bleeding compared to irreversible loss of brain tissue and neurological impairment associated with thromboembolic stroke warrants the need for further studies on individuals with an elevated stroke risk, who may obtain the most benefit with oral anticoagulation.38
Given the rapid growth of artificial intelligence (AI) in cardiovascular electrophysiology, our findings warrant the discussion of its role in AHRE. This is examined in a review by Harmon et al., who highlight the applicability of AI in the screening, detection and treatment of clinical AF.39 AI-based algorithms have already showed promise in predicting clinical AF based on electrocardiogram findings among patients who exhibit normal sinus rhythm.39 Furthermore, AI algorithms have demonstrated clinical utility in risk-stratifying patients for targeted screening of clinical AF, taking into account ECG findings and electronic medical record data, illustrating its potential benefit in patients with elevated stroke risk factors who are noted to have AHRE.39 Given that the decision to initiate anticoagulation in patients with AHRE is often unclear, AI can aid providers in the multifaceted and shared decision-making process involved in AF medical management.39 Limitations do, however, exist in the applicability of AI modalities for AF, with Harmon et al. citing external validation concerns, mixed perceptions among providers, and lack of availability to socioeconomically disadvantaged patients.39 Nonetheless, as AI technology continues to grow and be refined, we anticipate the clinical applicability of AI algorithms will have a tremendous impact in transcending management of patients with AHRE.39
Limitations
There are several limitations present in our meta-analysis. First, while individuals in our meta-analysis did not have a prior documented history of AF, this does not exclude the possibility that they have had undiagnosed or paroxysmal AF not known to them or their providers. Second, the fact that these individuals possessed severe cardiac comorbidities warranting the need for CIEDs brings into question whether these comorbidities may have played a role in the amplified risk of arrhythmias and thromboembolism. Third, the timespan of outcomes assessed is another limiting factor as certain studies ended prematurely, which may have prevented the effects of the interventions from truly being assessed. Other limitations of our meta-analysis include variability in the definitions of AHREs and outcomes between the individual studies. While the AHRE detection time ranged between 5 and 6 minutes in each of the trials included in our meta-analysis, AF, stroke and mortality may be more likely in individuals with longer episodes.
Conclusion
In this meta-analysis, AHREs in individuals with no prior history of AF were associated with a significantly increased risk of developing clinical AF and thromboembolism. However, there was no significant association noted with all-cause mortality. Further RCTs are indicated to verify our findings, and this meta-analysis should serve as a guide for future studies regarding AHREs in individuals with no prior history of AF. 
Click here to view Supplementary Material
Clinical Perspective
- Outcomes of subclinical AF in patients with no prior history of AF remain unclear.
- Our meta-analysis included 7,440 patients with subclinical AF.
- Patients with subclinical AF were likely to develop clinical AF.
- Subclinical AF was associated with an increased risk of thromboembolism.
- Duration of subclinical AF may impact the risk of thromboembolism as episodes ≥24 hours were associated with an increased thromboembolic risk while episodes <24 hours were not.