AF is the most common sustained cardiac arrhythmia and is seen as a health epidemic.1 In Europe, one in three individuals aged ≥55 years will develop AF at some point in the future.2 AF-related strokes account for approximately one-quarter of all strokes.3,4
Lifelong oral anticoagulation (OAC) remains the cornerstone of AF management, given it reduces stroke risk by two-thirds and mortality by one-quarter, but this is at the expense of increased major bleeding occurring.5–7 Real-world data suggest a 5% yearly risk of a major bleeding event but, if the predicted annual stroke rate is above 1%, instigating OAC has a net clinic benefit.6,8
Guidelines recommend using risk scores, such as the CHA2DS2-VASc, to inform anticoagulation decisions.9,10 However, these are based solely on clinical risk factors and do not include AF temporal patterns or burden.
A meta-analysis of almost 100,000 patients with AF showed that both the adjusted and unadjusted stroke and mortality risks are lower in paroxysmal AF.11 Similarly, the yearly stroke rate was below 1% in non-anticoagulated patients with AF paroxysms of <23.5 hours’ duration and a CHA2DS2-VASc score between 1 and 2, suggesting a lower stroke risk in association with short AF episodes.12
The justification for continuous, long-term OAC in such patients appears to be weaker and, furthermore, indefinite OAC may expose patients with short or infrequent AF episodes to a high bleeding risk relative to the more limited benefit in stroke reduction.
A tailored or ‘pill-in-the-pocket’ OAC strategy is based upon the concept that the thromboembolic risk is dynamic in that it increases during and shortly after an AF episode and then decreases during periods of sinus rhythm. With the advent of direct oral anticoagulants (DOACs), appropriate anticoagulation is established in just a few hours and no monitoring or dose adjustments are required.
Limiting OAC to periods of AF in carefully selected patients with low stroke risk and infrequent episodes of AF may offer the same thromboembolic protection as continuous OAC, while reducing healthcare costs and bleeding complications, and potentially improving adherence.
The aim of this study was to systematically review the literature to determine the feasibility, safety and efficacy of pill-in-the-pocket OAC.
Methods
Search Strategy
This systematic review was performed according to the Preferred Reporting Items Systematic Reviews and Meta-Analysis (PRISMA; Supplementary Table 1) statement and registered in PROSPERO (CRD42020209564).13
The search strategy was designed and conducted with the assistance of an experienced research librarian using MeSH terms and keywords to search Medline and Embase databases from inception to July 2022 (Supplementary Tables 2 and 3). We conducted a manual review of reference lists, editorials and review articles to identify secondary source documents not captured by the initial database search.
Study Selection and Data Extraction
Studies were included if a daily rhythm monitoring strategy (intermittent or continuous) was employed to identify AF episodes and guide OAC decisions in patients with a previous diagnosis of AF. The exclusion criteria were: patients with valvular AF; case reports, conference proceedings, commentary and letters; and editorials and review articles, but their reference lists were manually searched.
The result of the initial database search was exported to EndNote X7.0.1 and duplicate citations were removed. Two independent investigators (ABG and MP) then proceeded to screen the remaining abstracts and, if appropriate, full-text manuscripts against the eligibility criteria. Any discrepancies were resolved by a third investigator (TB). A standardised data extraction form was used.
The outcomes of interest were defined a priori as: OAC use or time on OAC; all-cause mortality; thromboembolic events; major bleeding; minor bleeding; and intracranial haemorrhage.
The methodological quality of the studies was examined using the Cochrane tool for assessing risk of bias for randomised controlled trials (RCTs) and the Robin-I tools for non-randomised research. Each study was independently reviewed by two investigators (ABG and MP) and any disagreements were decided by the senior author (TB). Funnel plots were produced to assess publication bias for the outcomes of interest.
Data Analysis and Synthesis
Continuous variables were summarised by extracting mean and standard deviation, if available, or calculated from the reported medians and interquartile ranges. Categorial variables were expressed as frequencies and percentages.
Outcomes of interest were extracted from each individual study and the event rate per year of follow-up was estimated. If the total follow-up duration was not available, it was calculated by multiplying the number of patients by the mean follow-up time in years. All outcome data were pooled using an inverse variance random-effect model as described by DerSimonian and Lard, producing a summary estimate of event rate per year of follow-up with an accompanying 95% confidence interval (CI).14
If studies reported zero outcome events, a continuity correction of 0.5 was applied. Heterogeneity was assessed by using Cochran’s Q test and its magnitude was quantified using the I2 statistic. Significance was set at 0.05 and all analyses were two-sided. Statistical analysis was performed using R statistical software version 4.0.3 and the metafor and meta packages (www.r-project.org).
Results
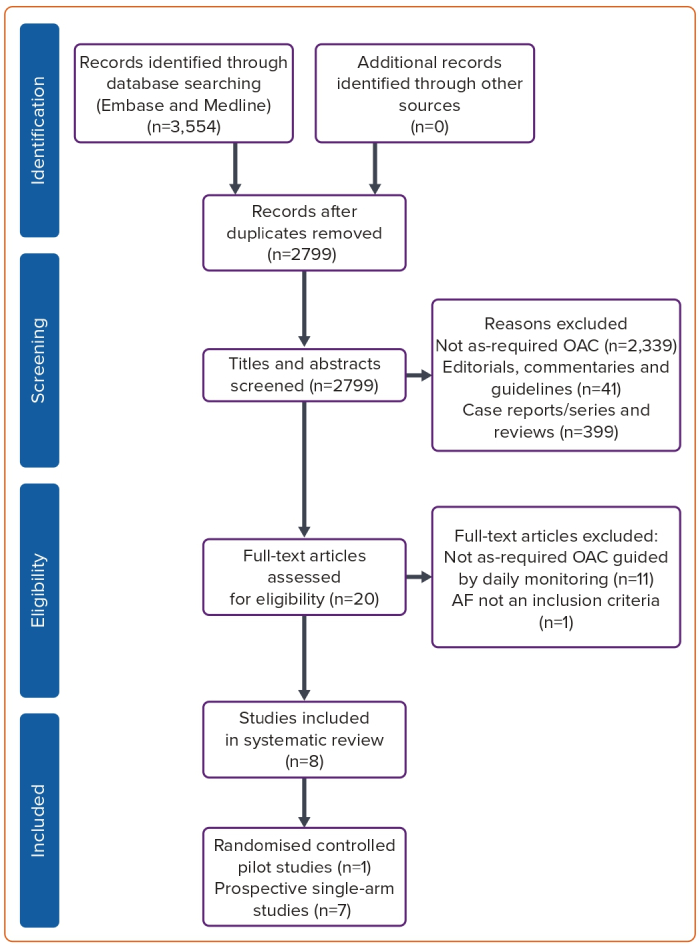
The Embase and Medline literature search identified 2,799 unique publications. Of these, 2,779 were excluded after screening the title and abstract. The remaining 20 full-text publications were reviewed for eligibility; only eight studies met the inclusion criteria and were included for review (Figure 1). Seven studies were prospective studies without a control group, and one was a randomised controlled pilot study. A summary of the included studies is displayed in Table 1 and baseline characteristics in Supplementary Table 4.15–22
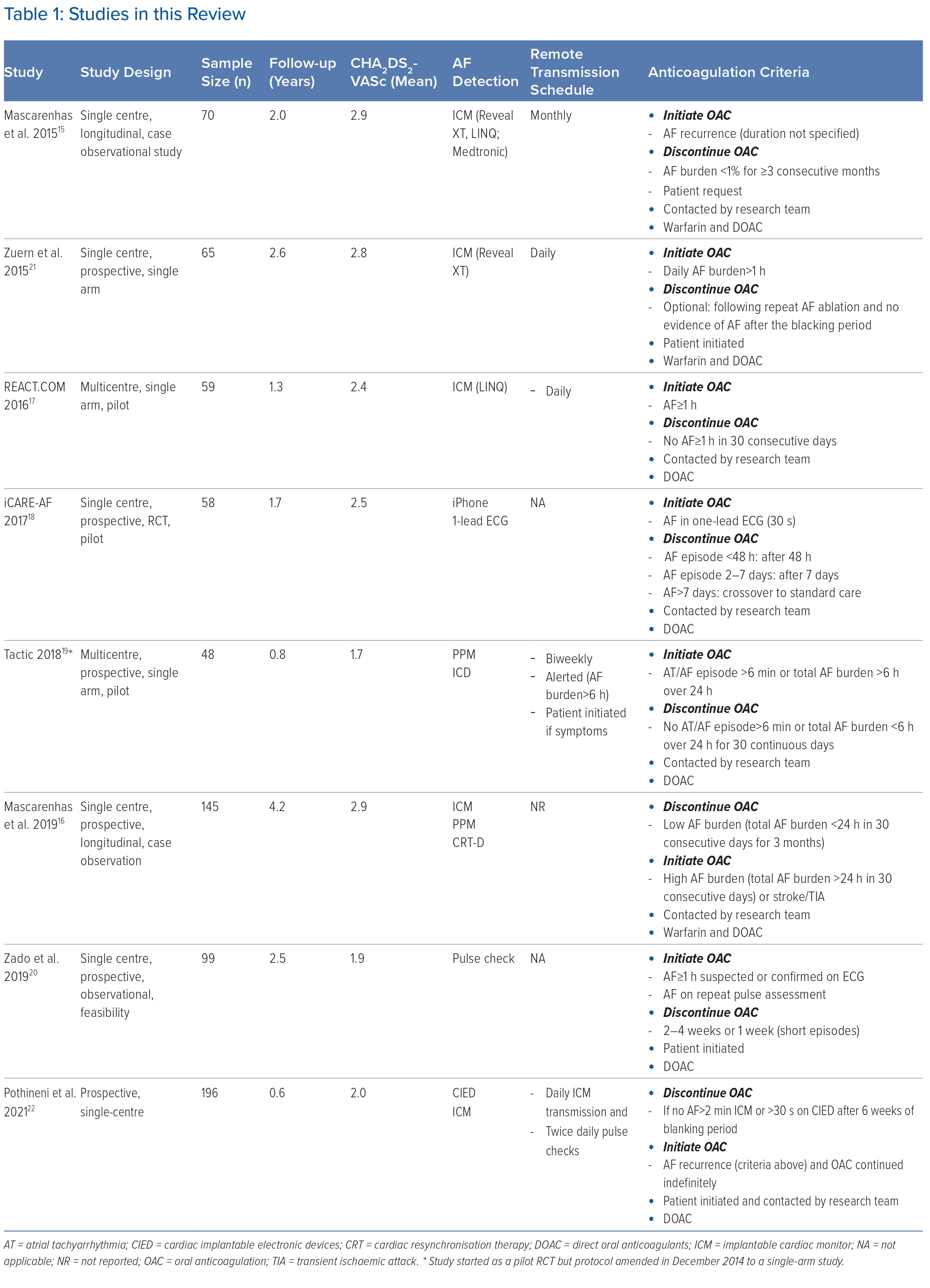
Overall, 711 patients were on a pill-in-the-pocket OAC strategy guided by daily rhythm monitoring. Follow-up duration ranged from 0.8 to 4.2 years with a total of 1,396 years of patient follow-up. The mean age was 68.3 years, 67% were men, 73% had paroxysmal AF and the mean CHA2DS2-VASc score was 2.2.
Study quality ranged from an overall low risk of bias in REACT.COM and Zuern et al. to moderate in the remaining studies (Supplementary Tables 5 and 6).17,21 Examination of funnel plots did not reveal any significant concerns regarding publication bias (Supplementary Figure 1).
Rhythm Monitoring Strategies and Oral Anticoagulation Criteria
Two studies (128 patients) used intermittent rhythm monitoring (spot-ECGs and pulse checks) and, in six studies (583 patients), continuous rhythm monitoring was employed with implantable cardiac monitors (ICMs) or cardiac implantable electronic devices (CIEDs) with an atrial lead.15–17,19,21,22 Criteria used to initiate and discontinue OAC were heterogenous, perhaps reflecting technical constraints of the devices used and the uncertainty surrounding AF episode duration that warrants anticoagulation (Table 1).
None of the studies reported the mean time from AF episodes detection to patients starting OAC. Only three studies had patient-initiated OAC.20–22 Zado et al. instructed patients to take OAC if pulse assessments revealed AF lasting 1 hour or short, frequent episodes.20 Pulse checks were performed only twice daily and asymptomatic episodes may have been missed. Similarly, in the study by Pothineni et al., participants performed twice-daily pulse checks but the duration that would trigger OAC was not described.22 In Zuern et al., patients performed daily manual ICM interrogation with a hand-held device that alerted them to take OAC.21 The AF burden was counted every calendar day so there may have been delays but it is likely that, if the study protocol was complied with, OAC was started within 24 hours.
The remaining five studies had three rate-limiting steps: remote transmissions; adjudication of all episodes; and, if appropriate, contacting patients.15–19 Complexity of remote transmission adjudication may have led to further delays. Investigators in iCARE-AF reviewed 30-second single-lead ECGs and patients started OAC within 1 hour of adjudication.18
Oral Anticoagulation Use
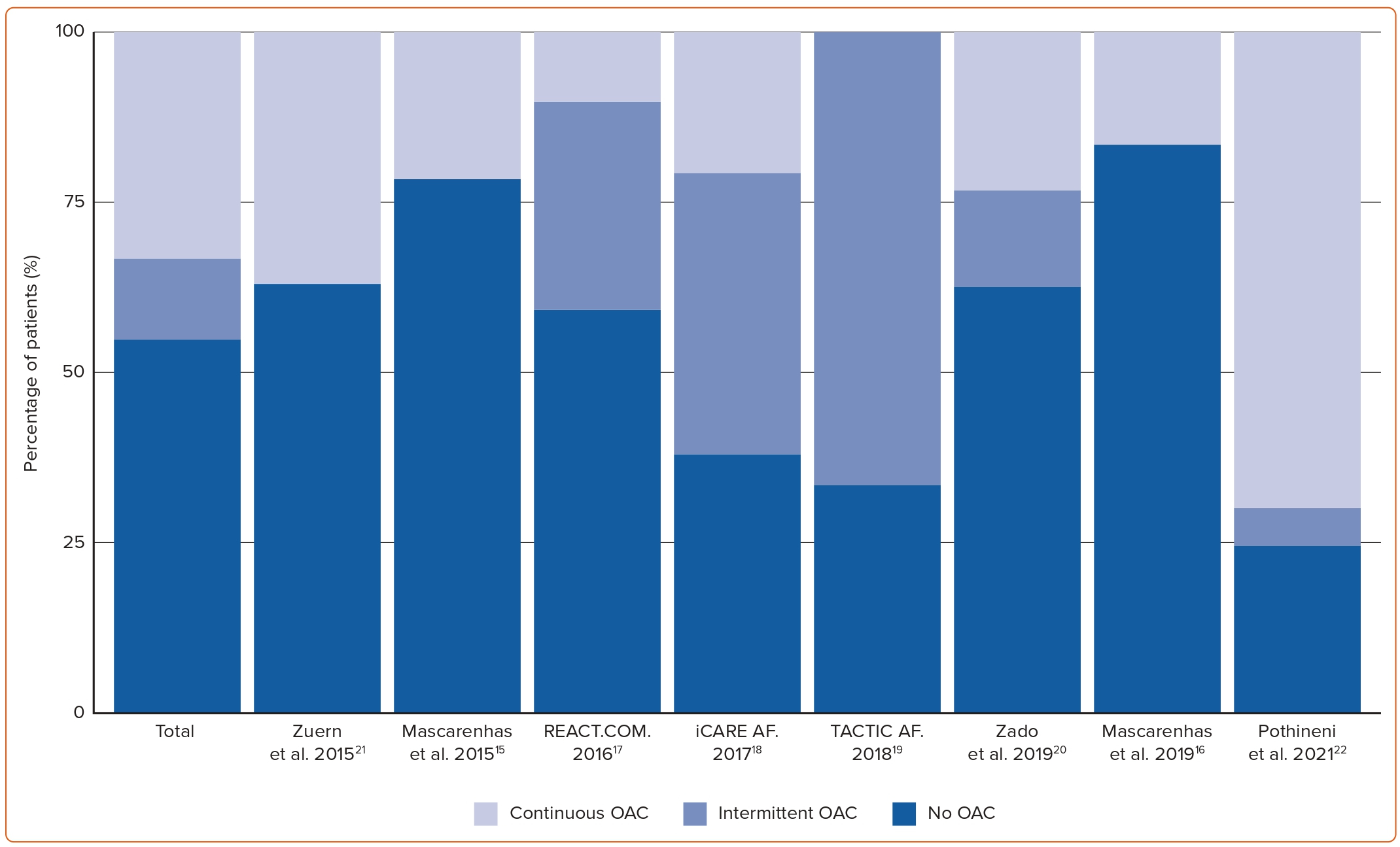
Overall, adopting a pill-in-the pocket strategy led to 390 (54.7%) patients not taking OAC during the study period and 85 (12.0%) patients taking it only intermittently following AF episodes (Figure 2). The remaining 237 (33.3%) patients remained on or returned to continuous OAC, mostly because they had a high AF burden or permanent AF. Other reasons included adverse events (three transient ischaemic attacks (TIAs) and one ischaemic stroke), other indications (13 patients), non-compliance with protocol (four patients) and patients’ wishes (four patients).
Four studies assessed the total duration of anticoagulation. REACT.COM, TACTIC-AF, Zuern et al. and Pothineni et al. reported 94%, 75%, 60% and 20% reduction in time on anticoagulation from a pill-in-the-pocket strategy compared to standard of care (continuous OAC), respectively.17,19,21,22 However, inappropriate anticoagulation owing to a protocol violation in TACTIC-AF accounted for almost half of the time spent on OAC.19
Safety and Efficacy Outcomes
Thromboembolic Events
Neurological events were identified in only two studies and independently adjudicated in three.17–19 Only one patient (0.1%) had an ischaemic stroke (Supplementary Table 7); this was an 81-year-old man with a CHA2DS2-VASc score of 3 who was not taking OAC as he denied any pulse irregularity during pulse checks.
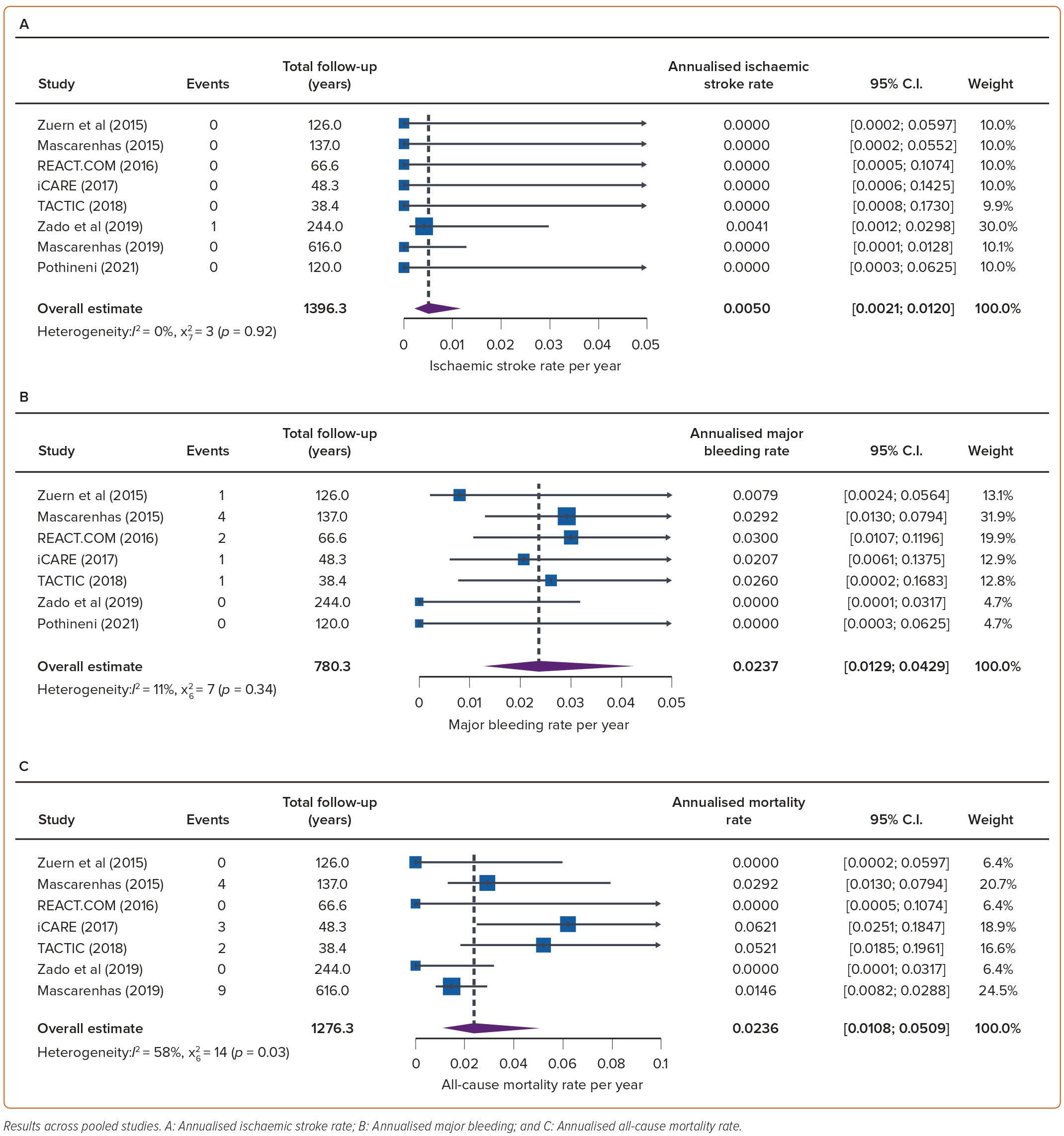
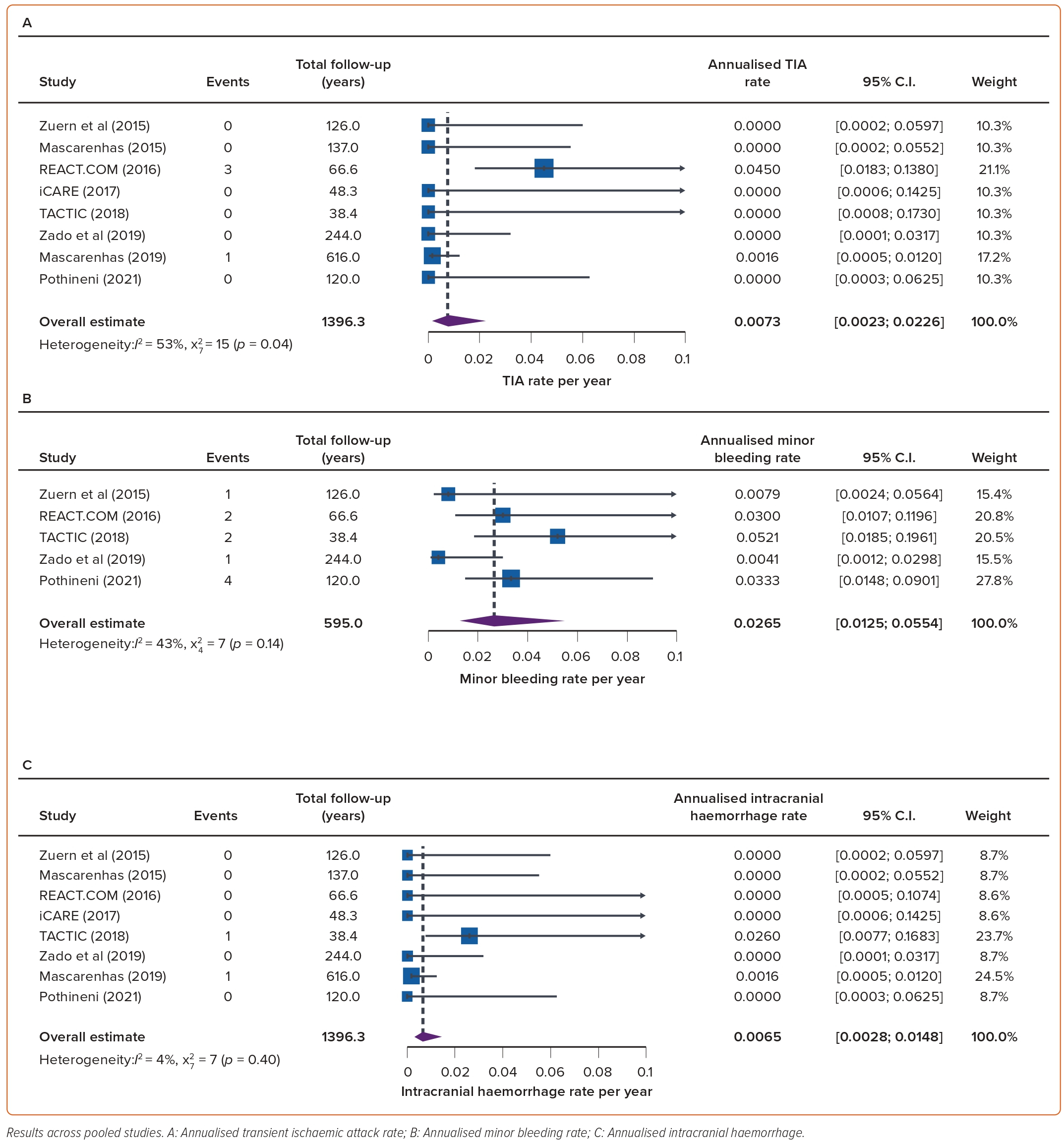
The overall annualised rates of ischaemic stroke and TIA were 0.005 (95% CI [0.002–0.012]) and 0.007 (95% CI [0.002–0.023]) per patient-year of follow-up, respectively (Figures 3–4). High heterogeneity (I2=57%, p=0.04) was observed only in TIA. No systemic embolism events were reported.
Bleeding Complications
Major bleeding was reported in seven studies (566 patients).15,17–22 There were nine (1.6%) major bleeding episodes over 780 patient-years of follow-up, representing an annualised major bleeding rate of 0.024 (95% CI [0.012–0.043]) per patient-year of follow-up.
One study reported bleeding events in 12 (8.5%) out of 145 patients, including nine gastrointestinal bleeds, but it is unclear whether these were major or minor bleeding episodes.16 Two intracranial haemorrhages were reported, both in patients not taking OAC, corresponding to an overall rate of 0.0065 (95% CI [0.0028–0.00148]) per patient-years of follow-up (Figure 4).
All-cause Mortality
Of the 515 patients in seven studies on pill-in-the pocket OAC, there were 18 deaths from any cause. The annualised mortality rate ranged from 0 to 0.0621 with an estimated overall 0.024 (95% CI [0.018–0.051]) deaths per patient-year of follow-up.
We found moderate between-study heterogeneity (I2=58%). Of the 18 deaths, four (22.2%) were classified as cardioVAScular (three from heart failure and one following an intracranial haemorrhage) and 14 (77.8%) as non-cardioVAScular. Pothineni et al. did not explicitly report the total number of deaths.22
Discussion
The main findings of this systematic review are:
- Criteria for initiation and discontinuation of OAC varied widely, reflecting the uncertainty regarding AF episode duration/AF burden and increase in thromboembolic risk.
- The mean time from AF detection to initiation of OAC was not reported and only three studies had patient-initiated OAC.
- In carefully selected patients with known AF and during a relatively short follow-up, the pill-the-pocket OAC strategy led to 54.9% of patients never restarting OAC and 12% of patients taking OAC only intermittently.
- Annualised stroke rate (0.5% (95% CI [0.2–1.2%]) was low but the small number of patients, short follow-up and lack of control groups precludes any conclusions regarding the safety and efficacy of a pill-in-the-pocket strategy.
The concept of pill-in-the-pocket OAC is a potentially attractive alternative strategy for a subset of patients with infrequent AF episodes and low stroke risk who would otherwise be committed to lifelong anticoagulation with all its implications.
The main premise for this strategy is the causal link between AF and ischaemic stroke: AF promotes blood stasis and thrombus formation which embolises to the cerebral arteries. The evidence for this direct relationship is, however, contradictory, particularly because there is a temporal disconnect between AF and stroke in patients with continuous rhythm monitoring, and this is still a matter of debate.23,24
Other essential unanswered questions are: How quickly does anticoagulation need to be initiated to prevent left atrial thrombus? How long does thromboembolic risk last following an AF episode? How to best monitor and alert patients during AF episodes?
AF and Stroke: Correlation or Causation?
The association between AF and thromboembolism is well-established in longitudinal studies and large clinical trials.5,7,25 Thrombi predominantly occur in the left atrial appendage. Closure of the appendage, either surgically or percutaneously, prevents ischaemic strokes; this suggests that in many patients, but not all, the mechanism of stroke is thromboembolic.26–28 Similarly, anticoagulation in the peri-cardioversion period is effective in reducing thromboembolic events.29,30
Arguments for AF being simply a marker of atrial myopathy rather than the cause of stroke stems from observations that atrial size, function and fibrosis are associated with stroke even in the absence of AF.31–34
Moreover, temporal dissociation between arrhythmic episodes and thromboembolic events has also been widely reported. Notably, only four (7.8%) out of 51 ischaemic strokes in ASSERT and six (13%) out of 69 thromboembolic events in IMPACT were preceded by AF.35,36 A much larger retrospective study of almost half a million patients with CIEDs had similar findings: only one-third of the 891 people who had ischaemic strokes had AF in the preceding 120 days.37
Undoubtedly, our current understanding is incomplete; arrhythmia alone is not responsible for all ischaemic strokes – a complex interplay of several factors is likely.
Temporal disconnect may be explained by other stroke mechanisms, such as large-artery atherosclerosis or hypertension-induced small-vessel occlusion. A CHA2DS2-VASc score ≥4 is an independent predictor of stroke in patients without AF; many patients with AF have competing VAScular risk factors for stroke.
Nonetheless, in a lower-risk population, arrhythmia may play a dominant role. Kaplan et al. demonstrated that, in patients with a CHA2DS2-VASc score of between 2 and 4, the thromboembolic risk increased with longer AF episodes, but those with a CHA2DS2-VASc score of ≥5 had a high event rate even without AF.12
Similarly, Botto et al. found a relation between CHADS2 score, AF duration and stroke risk.38 Patients with a CHADS2 score of 1 had low thromboembolic events only if AF duration was <24 hours. Higher-risk groups included patients with a CHADS2 score of 2 with AF duration of >5 minutes and those with a CHADS2 score of 3 with no AF.
Further evidence of the role of AF patterns can be seen in case-crossover studies which isolate AF patterns as the variable of interest; patients act as their own controls and the absolute risk derived from their clinical risk factors remains unchanged.
Studies by Turakhia et al. and Singer et al. showed a threefold increase in the relative risk of stroke in patients with a daily AF burden of >5.5 hours within 1 month of the event.37,39 The risk was greatest within 5 days of AF, then rapidly declined and returned to baseline after 1 month. Both studies strengthen the case for a causal link between AF episodes and stroke and suggest a transient increase in stroke risk following AF episodes, irrespective of baseline risk factors.
AF Burden and Thromboembolic Risk
The next challenge in delivering pill-in-the-pocket OAC is identifying the AF duration threshold that warrants anticoagulation and how quickly therapy should be initiated. It is biologically implausible that a universal cut-off exists or that the relationship between AF duration and stroke risk is linear. Nonetheless, it may be possible to identify a range of AF burdens associated with a steep increase in thromboembolic risk. Available data is conflicting, which explains the discrepancies in anticoagulation criteria used in the studies included in this review.
Guidelines suggest it is safe to proceed with cardioversion without excluding an intracardiac thrombus in AF episodes lasting <48 hours, implying that only longer episodes create a prothrombotic environment.9,10 There are, however, no RCT data to support this practice.
The assertion that there is a 48-hour window has been questioned by studies using long-term continuous rhythm monitoring. Large observational studies have shown an increased risk, with AF episodes ranging from 5 minutes in the MOde Selection Trial (MOST) and a daily burden of 5.5 hours in TRENDS to 24 hours in studies by Botto et al. and Capucci et al. as well as in post-hoc analysis of ASSERT.38,40–43 From these data, it is difficult to discern a clear cut-off for anticoagulation, but most studies suggest multi-hour episodes.
Two ongoing RCTs (NOAH-AFNET 6 [NCT02618577] and ARTESiA [NCT01938248]) of OAC in patients with device-detected AF may further inform our understanding.
The total duration of OAC required following an AF episode is also uncertain, but cardioversion data provide important insights. Conversion from AF to sinus rhythm is followed by a period of mechanical dysfunction of the left atrium and appendage, also known as atrial stunning; this leads to blood stasis despite organised atrial electrical activation, which contributes to increased thrombotic risk in the post-cardioversion period.44
The severity and recovery of atrial function depends on the duration of the preceding AF episode; brief episodes are followed by a swift return to baseline function while this make take up to a month after longer episodes.44,45
Most thromboembolic events occur within 10 days of cardioversion; without anticoagulation, the 30-day thromboembolic rate is in a range of 5–7%, which is mitigated with anticoagulation to <1%.46–48 The conventional strategy of at least 4 weeks of OAC after cardioversion is advocated by international guidelines to cover this vulnerable period.9
Continuous rhythm monitoring by Turakhia et al. and Singer et al., as described above, showed that stroke risk returned to baseline after 1 month.37,39
Taking this together, it is likely that at least a month of OAC is required in a pill-in-the-pocket strategy. Such a strategy is therefore only suitable for patients with a low AF burden and infrequent AF episodes.
Where Do We Go from Here?
The litmus test for any new intervention is an adequately powered RCT with meaningful outcomes, such as thromboembolic events, major bleeding and all-cause mortality. However, current evidence is insufficient to adequately inform how to design and conduct such a trial.
More work is needed to delineate anticoagulation criteria, how quickly it should be started and for how long. Studies with long-term AF monitoring in this cohort, with advanced neuroimaging to define the stroke mechanism, are warranted.49
Continuous rhythm monitoring is essential to deliver pill-in-the-pocket OAC; patient-reported AF symptoms are often unreliable.50 Pulse palpation or even daily ECG checks are likely to miss AF episodes and do not provide reliable data regarding AF episode duration and burden and are not suited for a pill-in-the-pocket strategy.
The workflow in the five studies with continuous rhythm monitoring was suboptimal and lacks scalability.15–17,19,21 Episodes were transmitted at best only once daily, investigators had to adjudicate all episodes and patients had to be contacted. This requires huge manpower – REACT.COM had 24,000 transmissions from 59 patients – has high costs and will lead to delays in starting OAC.17
The ideal solution is a closed loop, with devices informing patients in real-time of AF episodes, to reduce the time between AF detection and initiation of OAC. Four conditions must be met. First, diagnostic accuracy must be high to reduce the likelihood of inappropriate anticoagulation. Second, devices must communicate regularly and reliably with patients’ smartphones to deliver alerts. Third, devices must have a shared ecosystem with electronic health records/remote monitoring platforms to ensure physicians can adjudicate episodes and are aware if OAC is initiated. Finally, devices must be safe and minimally invasive.
Wearable devices have limitations that reduce their potential value as an arbiter for the delivery of rhythm-guided OAC. Specifically, motion artefacts are common and are responsible for high rates of false-positive detections.51 Compliance is not well studied and is likely to be suboptimal as batteries need to be recharged frequently and patients may be reluctant to wear devices overnight. Lastly, most wearable devices are consumer-facing without any integrated physician platforms for remote monitoring and adjudication.
Leveraging advancements in ICM technology is a more attractive strategy. The new iterations have improved diagnostic accuracy, particularly for longer AF episodes, connect to patients’ smartphones via low-energy Bluetooth and transmit device information to remote monitoring platforms with robust cybersecurity.52,53
ICMs have a miniaturised profile and are minimally invasive. The SMART-ALERT study (NCT05207150) is investigating the accuracy of closed-loop alerts from ICMs and two wearable devices (Apple Watch and CART-I ring) during episodes of AF and how promptly patients respond to these alerts.
Limitations
The present analysis is limited by the small number of patients included in most studies and notable heterogeneity in study methodology, baseline characteristics and criteria for both initiation and discontinuation of OAC, and the lack of a comparator.
A pill-in-the-pocket approach relies on swift initiation of OAC; however, none of the studies report the mean time from AF detection to patients starting OAC, raising doubts regarding the feasibility of the monitoring strategies. Studies without continuous rhythm monitoring may underestimate AF recurrence and thus OAC usage.
Moreover, observational studies have inherent biases and adverse events may be less rigorously collected than in a RCT and underreporting would affect overall event estimates. Nonetheless, this review provides a useful overview of different rhythm monitoring strategies, their pitfalls and applicability.
IMPACT did not meet the inclusion criteria as only 12% of patients recruited had AF and it was therefore not included in this review.36 It showed no difference in the primary endpoint (composite of stroke, systemic embolism and major bleeding) between standard care and OAC guided by atrial tachycardia monitoring and was stopped early due to futility. This RCT has limitations: it recruited predominantly patients with poor ejection fraction and with a high number of comorbidities (median CHA2DS2-VASc score 4), the OAC algorithm was complex and compliance was poor. Vitamin K antagonists were used by most patients (80.9%), raising concerns regarding inadequate anticoagulation due to its slow onset of action, prothrombotic effects during initiation and narrow therapeutic window.
Conclusion
A pill-in-the-pocket OAC strategy guided by daily rhythm monitoring challenges our current framework of stroke prevention in AF but evidence, although encouraging, is insufficient to inform practice.
To assess the risk:benefit ratio of pill-in-the-pocket OAC, an adequately powered RCT comparing it to continuous OAC (standard of care) in patients with infrequent AF episodes and low-to-moderate stroke risk is needed.
It is premature to proceed without additional studies that inform our understanding of the relationship between AF burden and thromboembolic risk and help delineate anticoagulation criteria and how to best deliver this therapy.
Click here to view Supplementary Material.
Clinical Perspective
- ‘Pill-in-the-pocket’ oral anticoagulation (OAC) is based upon the concept that the thromboembolic risk is dynamic; it increases during and shortly after an AF episode and then decreases during periods of sinus rhythm.
- In carefully selected patients with low CHA2DS2-VASc scores and infrequent AF episodes, targeted OAC during periods of AF may offer similar thromboembolic prevention as uninterrupted OAC while lowering bleeding complications and healthcare costs.
- Eight small pilot/feasibility studies (711 patients) showed a reduction in OAC use and a low annualised stroke rate (0.5% (95% CI [0.2–1.2%])). However, heterogenous OAC criteria, the small number of patients who had only a short follow-up, and the absence of control groups preclude any conclusion regarding the safety and efficacy of this strategy.
- Although increasing AF burden has been associated with higher stroke risk, the threshold that warrants OAC, how quickly it should be initiated and for how long are still uncertain. Further studies are needed to understand the relationship between AF burden and thromboembolic risk.