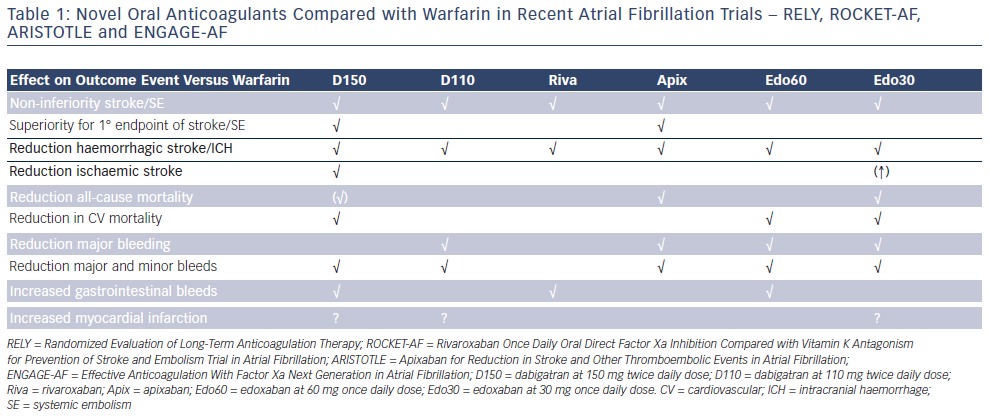
The introduction of novel oral anticoagulants (NOACs) has widened the treatment options for oral anticoagulation in stroke prevention in non-valvular atrial fibrillation (AF). Guidelines for the management of non-valvular AF have changed to reflect the emerging evidence of their relative safety and efficacy compared with warfarin (see Table 1).1–6
NOACs are now licensed for stroke prevention in patients with nonvalvular AF in many countries around the world as an alternative to vitamin K antagonists (VKAs). Recent guidelines incorporating the NOACs often refer directly or indirectly to the augmented CHADS2 score or CHA2DS2-VASc score, advising that other non-CHADS2 stroke risk factors (including age 65–74 years, female gender and vascular disease) may also influence choice and combine to favour a decision to initiate anticoagulation.
What Do Recent Guidelines Say?
The 2012 American College of Chest Physicians guidelines suggest the use of dabigatran 150 mg twice daily rather than warfarin where an oral anticoagulant (OAC) is recommended (i.e. for patients with a CHADS2 = 1 or CHADS2 ≥2). Back-up dual antiplatelet therapy may beconsidered for patients unsuitable for OAC therapy.1 Only dabigatran is mentioned, as at the time of publication only dabigatran was licensed in North America for stroke prevention in AF.
The 2012 Canadian Cardiovascular Society focused guideline update suggests that when OAC therapy is indicated, most patients should receive dabigatran or rivaroxaban in preference to warfarin (i.e. forpatients with a CHADS2 = 1 or CHADS2 ≥2).2
The 2012 American Heart Association/American Stroke Association Science Advisory recommend for patients with a CHADS2 ≥1, dabigatran 150 mg twice daily as an alternative to warfarin in renally competent patients, or apixaban 5 mg twice daily in patients considered appropriate for warfarin but who have no more than one of the following characteristics:
- weight <60 kg;
- age >80 years; and
- serum creatinine >1.5 mg/dl (i.e. who did not require the dose reduction to 2.5 mg twice daily).
For patients with a CHADS2 score ≥2, rivaroxaban 20 mg daily is considered a reasonable alternative to warfarin.3
The 2013 Scottish Intercollegiate Guidelines Network (SIGN) guidelines recommend that patients with non-valvular AF who have a CHADS2 or CHA2DS2-VASc score of ≥1 should consider taking warfarin or a NOAC, taking into account patient preference; while antiplatelet therapy should only be considered where warfarin or one of the novel anticoagulants has been declined. The SIGN guidelines are less specific in suggesting which NOAC is preferred, although they recognised that all the NOACs have been approved by the Scottish Medicines Consortium.4
The 2012 European Society of Cardiology (ESC) focused guideline update recommends the use of the CHA2DS2-VASc score to assess the risk of stroke in AF patients. For patients with a CHA2DS2-VASc = 0, no therapy is advised, but if CHA2DS2-VASc = 1 or CHA2DS2-VASc ≥2 then OAC therapy is advised; preferably one of the NOACs (either dabigatran or one of rivaroxaban/apixaban) should be considered instead of warfarin based on their net clinical benefit. However, female AF patients aged <65 years who score CHA2DS2-VASc = 1 for beingfemale only, remain low risk and should not be actively considered for therapy. Again, these guidelines do not specifically recommend which NOAC as none of these drugs have been directly compared with each other in randomised trials.5
The 2013 Asia Pacific Heart Rhythm Society (APHRS) guidelines recommend that the CHA2DS2-VASc score should be used to assess the risk of stroke, advising no therapy for patients with a CHA2DS2-VASc = 0 or 1, if scoring only for being female. For patients with a CHA2DS2-VASc = 1 OAC therapy is indicated, preferring dabigatran or apixaban over rivaroxaban, and warfarin if required. For patients with a CHA2DS2-VASc ≥2 then dabigatran, rivaroxaban, apixaban or warfarin are suggested. Where warfarin is to be used, an international normalised ratio (INR) of 1.6–2.6 for patients ≥70 years of age is preferred, at least in some countries such as Japan.6
All Guidelines Have a Preference for Novel Oral Anticoagulants Over Vitamin K Antagonists
As antiplatelet therapy fades into the background, the NOACs are emerging as a favoured therapy. A number of reviews and analyses examining the effects of the NOACs have been published pointing to their clinical benefits in reducing stroke, systemic thromboembolism and mortality compared with VKAs.7–9 Indirect comparisons between NOACs have been made, but should be interpreted with caution until head-to-head trials are under way.10,11
Time in Therapeutic Range of the Vitamin K Antagonists
To understand the advantages of NOACs, we need to take a closer look at the VKAs, such as warfarin. Warfarin acts diffusely at the initiation and amplification stages of the coagulation cascade, inhibiting the enzyme vitamin K epoxide reductase complex subunit 1 (VKORC1), disrupting the post-translational modification of vitamin K-dependent proteins, and thereby the synthesis of the vitamin K-dependent coagulation factors II, VII, IX and X.12–14
VKAs are a widely used OAC for stroke risk reduction in patients with AF, but may be underused in this capacity.13 Establishing standard clinical monitoring schedules for AF patients is inherently problematic as VKA initiation regimens involve loading doses and periodic blood tests to maintain the INR and the patient’s overall time in therapeutic range (TTR), an index of INR control. For AF patients starting VKA therapy, poor TTR remains an independent risk factor for major bleeding,15 although even when within the therapeutic range VKAs can carry a bleeding risk.
INR control varies widely between clinical centres, affecting the treatment benefit of VKA therapy, although a target threshold TTR (approximately 58–65 %) helps to maintain the benefit of VKAs over antiplatelet therapy.16 While achieving good anticoagulation control in patients on VKAs is associated with a reduction in the risk of stroke, mortality is also reduced in those who have a TTR of 70 %.17 Indeed, a TTR of 70 % or greater would reflect high-quality VKA management; however, the TTR is often found to be lower in clinical practice.12 The limitations of VKAs are due to:
- a slow onset of action;
- a variable dose requirement influenced by pharmacogenetics (e.g. of common polymorphisms, or variant alleles coding for VKORC1); and
- a differential dietary vitamin K intake and drug–drug interactions that influence their pharmacokinetics or pharmacodynamics.
Hepatic dysfunction, changes in gut flora and alcohol intake may also contribute. Patient compliance is no doubt linked to the medicalisationof the patient’s lifestyle that VKA therapy imposes.12
Prior to the emergence of NOACs, treatment practices for stroke prevention in patients with AF had focused on the identification of ‘high-risk’ patients to be targeted for warfarin. However, numerous studies have shown that clinicians consistently failed to achieve the full treatment potential that oral anticoagulation could bring to those AF patients who stood to benefit.18
Furthermore, although the use of VKA therapy had increased since 1980, and the proportion of patients receiving no therapy has decreased, many patients with AF received either antiplatelet therapy (10–56 %, median 30 %) or no therapy (4–48 %, median 18 %). Thus, many patients at moderate or high risk for stroke were not treated according to guidelines.19 The benefits of oral anticoagulation over antiplatelet or no therapy are often highlighted within guidelines, but when we consider that patients on antiplatelet or no therapy may suffer bleeding rates comparable with those on VKAs,20 promoting their correct use becomes important.
European Society of Cardiology Guideline Supports Novel Oral Anticoagulants, Including Oral Factor Xa Inhibitors
As the NOACs become more readily available, we may expect to see a reduction in the unacceptable numbers of these AF patients at increased risk for stroke who, once passed over for oral anticoagulation, remained unprotected. The ESC 2012 guidelines promote the NOACs in non-valvular AF because they show non-inferiority compared with VKAs and are inherently safer as their performance in phase III studies have shown.21–23 Currently, the oral factor Xa inhibitors are generating significant interest in stroke prevention as they dominate the NOAC field of choice.
The oral factor Xa inhibitors have predictable pharmacokinetics that allow for fixed dosing. Half-life, bioavailability, metabolism and excretion may differ somewhat, but universal advantages of the approved NOACs include:
- rapid onset of action;
- lower likelihood of food–drug interactions;
- limited drug–drug interactions;
- predictable anticoagulant effect;
- no requirement for routine coagulation monitoring; and
- reduced risk of intracranial haemorrhage.24
When analysed together, the factor Xa inhibitors significantly reduce stroke, systemic thromboembolism and intracranial haemorrhage compared with warfarin in patients with AF.25 However, there are too few guidelines on how best to measure (and reverse) the in vivo activity in clinical settings.
Edoxaban is a New Oral Factor Xa Inhibitor
Edoxaban is a new specific direct inhibitor of factor Xa. Rapidly absorbed orally, it has a half-life of approximately 10–14 hours26 and is approximately 40 % renally excreted with an element of active tubular secretion.27 Exposure increases with renal dysfunction and low body weight.28 It is not affected by food, and drug concentrations appear to have a linear correlation with anti-factor Xa activity and other coagulation indices. It appears safe and well tolerated with no adverse events in doses up to 150 mg and has predictable, consistent pharmacokinetic and pharmacodynamic profiles, and dose-proportional plasma concentrations.27 As with other oral Xa antagonists, it is a substrate for permeability (P)-glycoprotein; the P-glycoprotein inhibitors amiodarone and verapamil could increase plasma concentration, extending its half-life.29
A phase II comparison study with warfarin for patients with non-valvular AF found twice-daily doses of edoxaban 60 mg or edoxaban 30 mg were associated with higher bleeding rates, whereas a once-daily edoxaban dose of 60 mg or 30 mg had a similar safety profile to warfarin.30
A randomised, double-blind study of edoxaban versus warfarin for treating symptomatic venous thromboembolism conducted on 8,292 adult patients with acute, symptomatic deep-vein thrombosis (n=4,921) of the lower limb or acute, symptomatic pulmonary embolism (n=3,319) trialled 60 mg edoxaban orally once-daily (30 mg if renal impairment, low body weight or potential drug interactions). The TTR (2.0–3.0) achieved for the warfarin group was 63.5 %. Edoxaban was confirmed to be non-inferior to warfarin (hazard ratio [HR] of 0.89; 95 % confidence incidence [CI], 0.70–1.13) in treating recurrent symptomatic venous thromboembolism and caused significantly less bleeding (HR 0.81; 95 % CI, 0.71–0.94).31
Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation–Thrombolysis in Myocardial Infarction 48 (ENGAGE AF–TIMI 48) was a large phase III randomised, double-blind and double-dummy trial comparing two exposure strategies of edoxaban to warfarin using a non-inferiority design. In total, 21,105 patients were randomised in a 1:1:1 ratio to edoxaban high exposure (60 mg daily), edoxaban low exposure (30 mg daily) or warfarin titrated to an INR of 2.0–3.0. Sham INRs in patients receiving edoxaban facilitated blinded treatment. The edoxaban exposure strategies of 60 mg and 30 mg allowed for dynamic dose reductions in subjects with anticipated increased drug exposure requiring dose adjustment according to drug clearance. Eligibility criteria included electrocardiographic verification of AF (paroxysmal, persistent or permanent) within 12 months of randomisation, in patients with a CHADS2 score ≥2. Randomisation was stratified by CHADS2 score (2–3 versus 4–6) and the clinical need for dose reduction of edoxaban. The primary objective was to determine whether edoxaban was non-inferior to warfarin for the prevention of stroke and systemic embolism and the primary safety endpoint was major bleeding. Rates of endpoints were represented for one year and the median follow-up was 2.8 years.28,32 The trial results, in essence, showed that edoxaban, when compared with warfarin, was non-inferior in the prevention of stroke or systemic embolism with significantly lower rates of bleeding and death from cardiovascular causes.32
Both dose regimens of edoxaban achieved significance in the non-inferiority analysis when compared with warfarin. The rate for the primary efficacy endpoint of stroke or systemic embolism in the on-treatment analysis was 1.50 % with warfarin (median TTR achieved, 68.40 %) compared with 1.18 % for edoxaban 60 mg (HR of 0.79; 97.5 % CI, 0.63–0.99) and 1.61 % for edoxaban 30 mg (HR of 1.07; 97.5 % CI, 0.87–1.31). High-dose edoxaban was significantly superior to warfarin in the on-treatment analysis. In the pre-specified intention-to-treat analysis, neither regimen of edoxaban was found to be superior to warfarin, although there was a trend favouring edoxaban 60 mg versus warfarin (HR of 0.87; 97.5 % CI, 0.73–1.04; P=0.08) over edoxaban 30 mg versus warfarin (HR of 1.13; 97.5 % CI, 0.96–1.34; P=0.10). For rates of ischaemic stroke only edoxaban 30 mg was associated with a significantly increased rate at 1.77 % (HR of 1.41; 95 % CI, 1.19–1.67), when compared with either edoxaban 60 mg or well-controlled warfarin, between which, ischaemic stroke rates were the same at 1.25 % (HR of 1.00; 95 % CI, 0.83–1.19; P=0.97).
The rates for the primary endpoint of major bleeding were significantly lower for high- and low-dose edoxaban compared with warfarin: 3.43 % with warfarin versus 2.75 % for edoxaban 60 mg (HR of 0.80; 95 % CI, 0.71–0.91) and 1.61 % with edoxaban 30 mg (HR of 0.47; 95 % CI, 0.41–0.55). The rate of haemorrhagic stroke was significantly higher with warfarin, 0.47 % as compared with 0.26 % for high-dose edoxaban (HR of 0.54; 95 % CI, 0.38–0.77) and 0.16 % for low-dose edoxaban (HR of 0.33; 95 % CI, 0.22–0.50).
The rates of cardiovascular death were significantly lower for both regimens of edoxaban, 3.17 % among the warfarin group versus 2.74 % (HR of 0.86; 95 % CI, 0.77–0.97) for edoxaban 60 mg and 2.71 % (HR of 0.85; 95 % CI, 0.76–0.96) for edoxaban 30 mg. Corresponding rates of the composite of stroke, systemic embolism or all-cause cardiovascular death were 4.43 % versus 3.85 % (HR of 0.87; 95 % CI, 0.78–0.96; P=0.005) and 4.23 % (HR of 0.95; 95 % CI, 0.86–1.05; P=0.32).32
Edoxaban Would Fit in Nicely with Current Guidelines
Edoxaban 60 mg would complement current guidelines on stroke prevention in non-valvular AF due to its consistency among the NOAC range in terms of its non-inferiority to warfarin, and its safety profile with respect to bleeding risk and intra-cranial haemorrhage; while it may have the concomitant benefit of reducing cardiovascular mortality. It comes as a once-daily dose with a patient-friendly profile in terms of its tolerance and ease of use, and may indeed present a clinician-friendly profile too, allowing the drug to be fitted to the patient should concerns of safety over the risk of bleeding outweigh efficacy in stroke prevention. By reducing the dose to 30 mg where there is anticipated increased drug exposure, edoxaban would remain non-inferior to warfarin in such patients.
Conclusion
With the availability of so many NOACs as alternatives to warfarin, we are now rather spoilt for choice, and we have the opportunity to fit the drug to the patient (and vice versa). As discussed above, good quality anticoagulation control with warfarin is associated with high efficacy and safety (with low stroke and bleeding risks), and thus, effective stroke prevention in guidelines essentially means the use of well-controlled warfarin (TTR ≥70 %) or one of the NOACs.
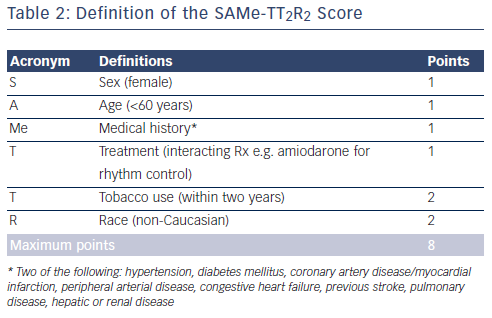
A decision dilemma is how to predict upfront those newly diagnosed non-anticoagulated AF patients who would do well on warfarin with high TTR, given costs of the new drugs and that the benefits of NOACs over warfarin may be only marginal in those with high TTRs. The new SAMe-TT2R2 score33,34 (see Table 2) may help with the decision-making process, as this is a new user-friendly validated simple clinical score, which identifies those AF patients likely to do well on warfarin (SAMe-TT2R2 score 0–1) or those more likely to have poor anticoagulation control (SAMe-TT2R2 score ≥2). An ESC position document12 recommends that AF patients with a SAMe-TT2R2 score >2 should be considered to be better off being started on NOACs as initial therapy, or have more aggressive efforts to improve anticoagulation control.