Ventricular tachycardia (VT) is associated with increased mortality in patients with a history of MI. ICD implantation is currently the standard of care for the prevention of sudden cardiac death, and contributes to a reduction of total mortality.1 Despite effective treatment of ventricular arrhythmias with the use of anti-tachycardia pacing (ATP) or shocks, ICDs do not prevent VT. Furthermore, sudden cardiac death still occurs in approximately 5% of ICD patients, and ICD therapies are associated with an increase in mortality and a reduction in quality of life.2–5
According to a recent meta-analysis, mortality after appropriate shock is higher than after inappropriate shock, suggesting an increased risk related to the underlying arrhythmia substrate.3 The concept of VT ablation aims at effective treatment of the underlying arrhythmia substrate with consecutive prevention of shocks instead of treatment of VT with the use of shocks. Early VT ablation, defined as ablation within 30 days after the first documented VT, has been associated with a lower VT recurrence rate, as compared with VT ablation performed later.6 Preventive catheter ablation before the occurrence of any ICD therapy might contribute to improved prognosis, compared with deferred VT ablation.
Recent guidelines recommend urgent catheter ablation in patients with scar-related heart disease presenting with incessant VT or electrical storm, and in patients with ischaemic heart disease and recurrent ICD shocks due to sustained VT.1 Furthermore, catheter ablation should be considered after a first episode of sustained VT in patients with ischaemic heart disease and an ICD.1 Data on preventive catheter ablation at the time of ICD implantation are limited, but potential benefits might relate to a reduction of ICD therapies and mortality. The aim of this meta-analysis was to assess the safety and efficacy of VT ablation prior to or at the time of secondary prevention ICD implantation in patients with coronary artery disease, as compared with deferred VT ablation.
Methods
Search Strategy
Studies were identified by searching electronic databases (Medline via OvidSP, Embase via OvidSP, Medline via PubMed, BIOSIS Previews via OvidSP, Web of Science and Scopus) from inception to 21 December 2017. The literature search used text words and relevant indexing to capture data on catheter ablation of VT/VF or arrhythmogenic substrate modification in patients with ischaemic cardiomyopathy undergoing ICD implantation. The following search structure was used in Medline and translated into the other databases as appropriate: (catheter ablation[title/abstract]) AND (implantable defibrillator[title/abstract]) OR (implantable cardioverter defibrillator[title/abstract]) AND (ventricular fibrillation[title/abstract]) OR (ventricular tachycardia[title/abstract]). Handsearching, with cross-references of retrieved publications, review articles and guidelines, was also performed to ensure that all relevant studies were included. No restriction on study type or language was applied.
Study Selection
Studies selected for inclusion were randomised trials that tested the safety and effectiveness of catheter ablation for VT before ICD implantation versus ICD implantation alone in patients with ischaemic cardiomyopathy. Cohort studies, case reports, case series, review articles and conference abstracts were excluded. Selection was limited to English-language articles. The first screening was performed independently by two authors (RP and CE), based on title and abstract. Next, the full text of the eligible articles was examined to ensure that they met the study criteria: randomisation of patients to catheter ablation plus ICD implantation versus ICD implantation alone, patients included had ischaemic cardiomyopathy, the catheter ablation strategy applied was described in detail, and data were provided regarding the safety and efficacy of both therapeutic approaches. From the selected studies, the same authors (RP and CE) then independently extracted data. Disagreements were resolved by discussion; if no accord was reached, a third author (RRT) made the final decision. Data on the type of study, year of publication, country where the study was performed, population included, presence of spontaneous or inducible VT/VF, type of ablation performed, duration of long-term follow up, the occurrence of appropriate ICD shock, appropriate ICD therapy (ATP and/or shock), arrhythmic storm, death and complications were extracted.
The primary outcome was the number of patients with an appropriate ICD shock in both groups at long-term follow up. Secondary outcomes were the number of patients with appropriate ICD therapy (shock and/or ATP), arrhythmic storm, death and major complications in the two groups.
Statistical Analysis
From the number of events extracted in the two groups (ablation plus ICD versus ICD alone) and their respective population, the number of non-cases in both groups was calculated. The OR was the primary measure of treatment effect or side-effect; 95% CIs for OR were calculated. The ORs were then pooled using a DerSimonian and Laird random effect model.7 Heterogeneity was assessed with I-squared statistics (I2). The I2 statistic indicated the percentage of variability due to between-study (or inter-study) variability, as opposed to within-study (or intra-study variability). An I2 >50% was classified as substantial presence of heterogeneity. The analysis was performed using commercially available software STATA 14 (StataCorp).
Quality Assessment
The risk of bias was assessed based on the tool from the Cochrane Collaboration.8 Specifically, the risk of bias was assessed in the following domains: selection (random sequence generation and allocation concealment), performance (blinding of participants and personnel), detection (blinding of outcome assessment), attrition, reporting and other bias. Every domain could be classified as high or low risk of bias. If the information reported in the article was insufficient, the domain was defined as unclear.
Results
Our literature search identified 340 citations after exclusion of duplicates. After review of the abstracts, nine of these references were considered potentially eligible for inclusion, and their full text was analysed in more detail. Three references were excluded because patients with non-ischaemic cardiomyopathy were included, one was excluded because of a lack of a comparison group and two were excluded because only patients with a prior ICD shock were included.9–14 Finally, a total of three randomised trials were included in the analysis (Supplementary Material Figure 1).15–17
Study Characteristics
The key features of the three trials are summarised in Table 1.15–17 All studies had a multicentre, prospective, randomised controlled study design.15–17 Randomisation was performed using computer-based 1:1 allocation (Ventricular Tachycardia Ablation in Coronary Heart disease; VTACH, Substrate Modification Study; SMS) or with the use of sealed numbered envelopes (Substrate Mapping and Ablation in Sinus rhythm to Halt Ventricular Tachycardia; SMASH-VT).15–17 Regarding drug therapy, patients taking class I or III antiarrhythmic medications were excluded from SMASH-VT; VTACH allowed antiarrhythmics in the control arm at the discretion of the treating physician, and SMS stratified the randomisation of patients according to treatment with amiodarone (30% of patients) or a beta-blocker.15–17
VT ablation was performed with the use of an electroanatomical mapping system (SMASH-VT, VTACH, SMS), a non-contact-mapping system (VTACH, SMS) and conventional ablation (SMS) with the use of irrigated and non-irrigated (SMASH-VT, VTACH, SMS) ablation catheters. In the SMASH-VT study, a non-irrigated ablation catheter was used throughout the study in the USA, as irrigated catheters had not been approved for clinical use until after the end of enrolment. Mapping and ablation was performed in sinus rhythm or, if AV conduction was not present, with ventricular pacing in SMASHT-VT, whereas patients in VTACH and SMS underwent mapping and ablation of stable VT or substrate modification in case of non-inducible or unstable VT.
In case of inducible VT at the beginning of the procedure, the ablation endpoint was non-inducibility of the clinical VT (SMS) or any VT (VTACH). In case of non-inducibility, the absence of all channels/late potentials inside the area of interest or ablation with linear lesions based on pace mapping along the infarct scar target sites was aimed at (VTACH) or the ablation strategy was based on the anatomy of the substrate, and the procedural endpoint was the implementation of the projected ablation lines (SMS). In the SMASH-VT study, the VT was induced, the arrhythmogenic portions were identified by pace mapping, and entrainment mapping and targeted for ablation with linear ablation lines or with ablation of the late and/or fractionated potentials deeper within the scar. If only VF or polymorphic VT was inducible, stimulation was repeated after intravenous infusion of a class I antiarrhythmic drug (procainamide or ajmaline).
The ICD manufacturer and programming differed between studies. While SMASH-VT did not standardise ICD manufacturers and programming, except the definition of at least one VT zone with ATP, VTACH only used St Jude Medical devices and SMS Medtronic devices with predefined programming (VF zone with a cut-off rate of 200–220 BPM, and a VT zone with a cut-off cycle length of 60 ms above the slowest documented VT and anti-tachycardia pacing followed by shock therapy).
Baseline characteristics of patients included in the three studies were quite similar and are shown in Table 2. Differences relate to the use of antiarrhythmic drugs at inclusion, with none of the patients in SMASH-VT receiving amiodarone, whereas about 30% of patients in the other studies were taking amiodarone.
Appropriate ICD Shocks
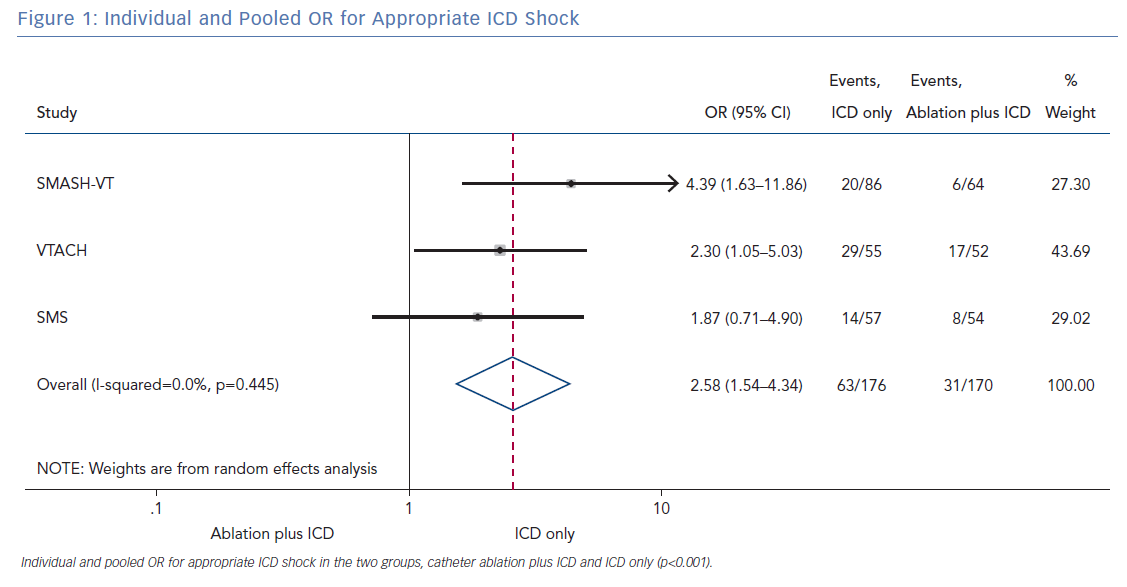
All three randomised trials reported data on the number of patients with an appropriate shock in the two groups (ablation plus ICD and ICD only). Overall, 346 patients were included in the analysis. The random effect pooled analysis showed an OR of 2.58 (95% CI [1.54–4.34]; p<0.001), favouring the former. The heterogeneity was 0.0%; p=0.445 (Figure 1).
Secondary Endpoints
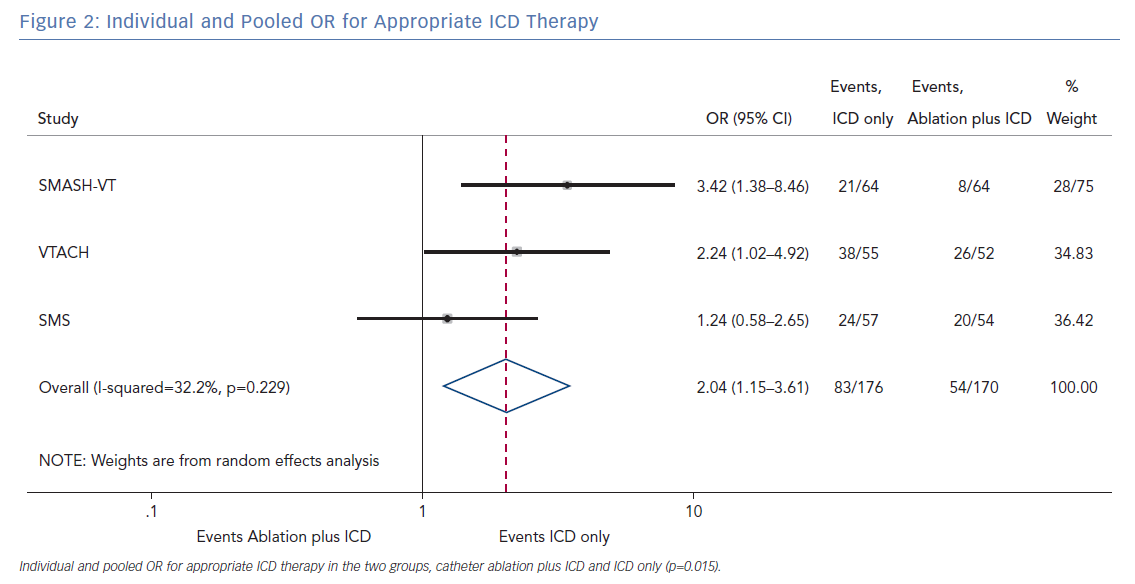
The pooled analysis on the endpoint of any appropriate ICD therapy included 346 from all three randomised trials. The cumulative OR was 2.04 (95% CI [1.15–3.61]; p=0.015), favouring ablation plus ICD. The I2 was 32.2%; p=0.229 (Figure 2).
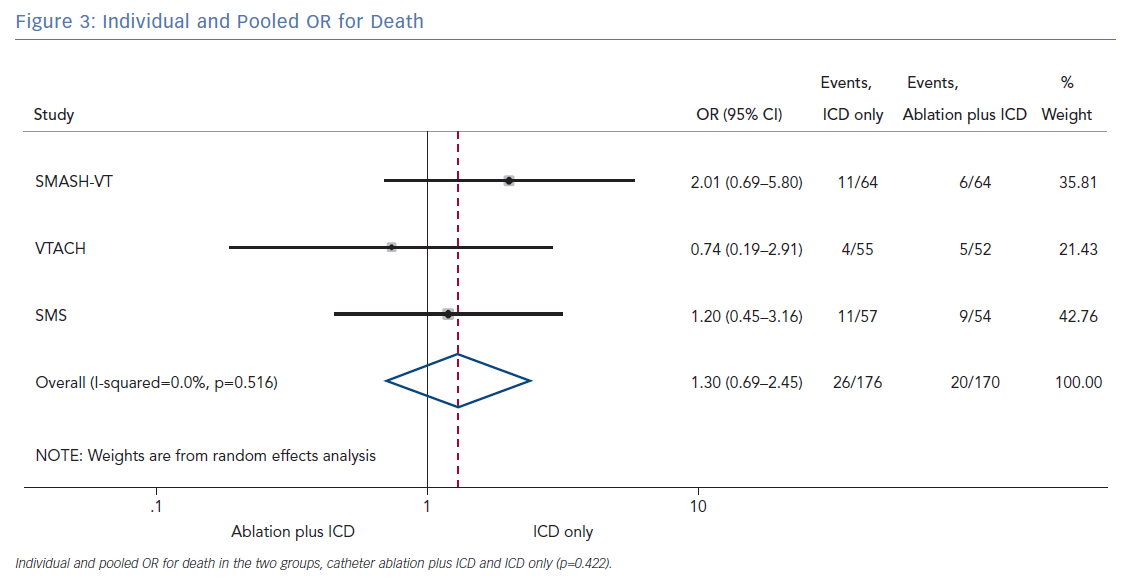
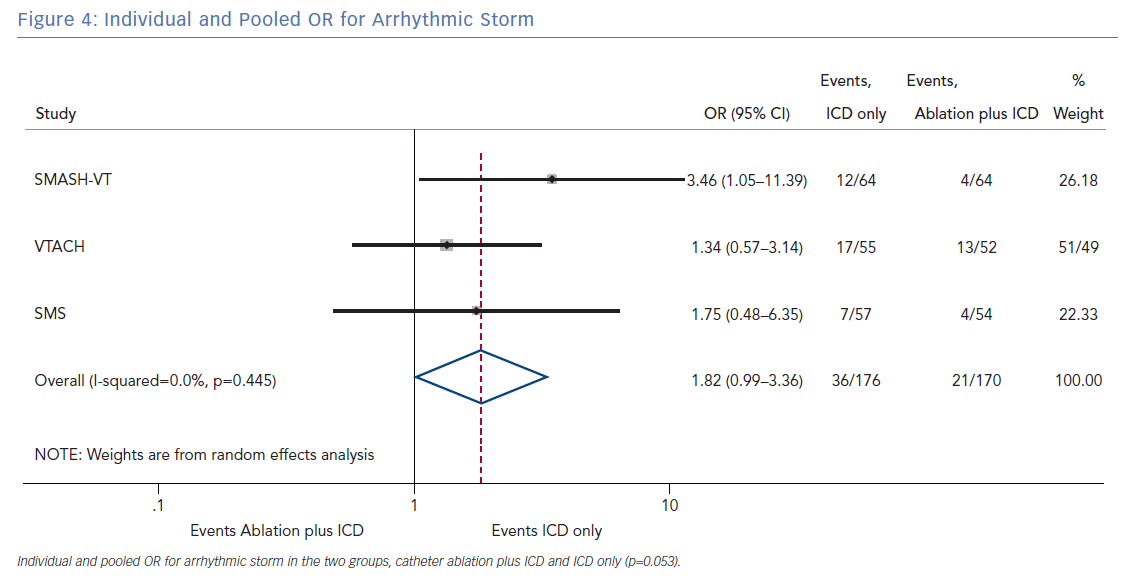
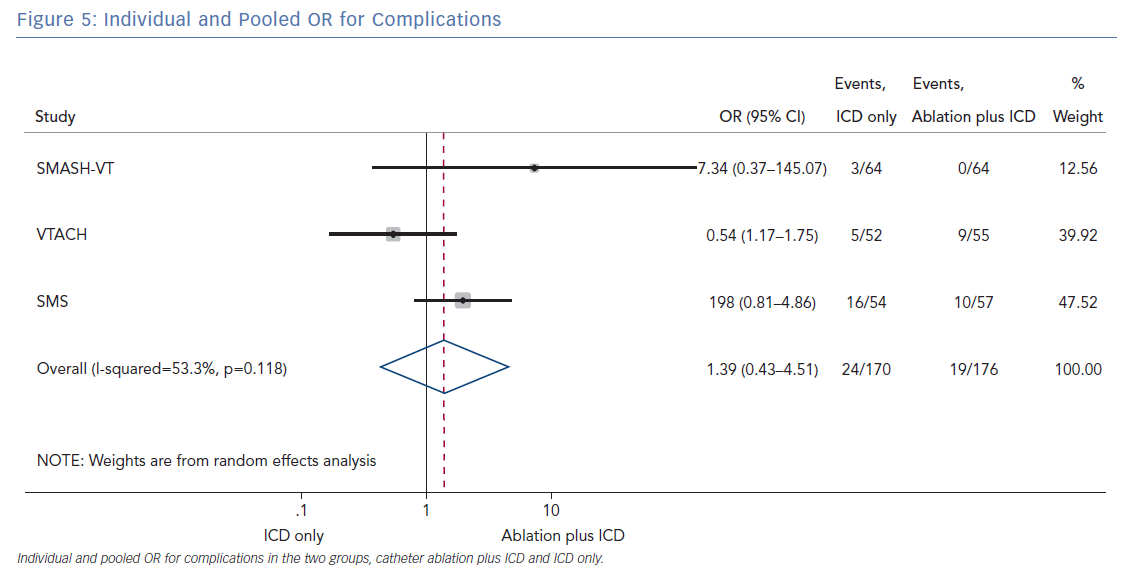
The number of deaths for both groups was reported in all three randomised trials for an overall number of 346 patients with a pooled OR of 1.30 (95% CI [0.60–2.45]; p=0.422; Figure 3). The number of arrhythmic storms was reported in two studies, whereas one study reported the percentage of patients free from arrhythmic storms at follow-up; from this, the number of patients with arrhythmic storms was calculated, the pooled OR of 346 patients was 1.82 (95% CI [0.99–3.36]; p=0.053; Figure 4). Complications were reported in all three studies with a pooled OR of 1.39 (95% CI [0.43–4.51]; p=0.581; Figure 5).
Quality Assessment
All three studies were judged by the investigators to be at high risk of bias in several items. Specifically, although all three studies were registered randomised trials, only VTACH and SMS used a computer processing sequence for randomisation. Patients in the SMS study were block randomised and subrandomised using the SAS software according to the following clinical characteristics: ejection fraction (EF) >30% versus EF <30%, amiodarone versus no amiodarone treatment and beta-blocker versus no beta-blocker treatment. Indeed, the generation of the randomisation sequence in the SMASH-VT trial was evaluated as suboptimal due to the use of envelopes.
Also, performance and detection bias have been judged at high risk in all three studies; considering the endpoints and the type of intervention, it may have been difficult to achieve blinding of the participants. However, none of the studies discussed or adopted measures to overcome this issue. Moreover, all of them had the same physicians performing the procedure and assessing long-term outcomes.
Both investigators agreed that the data collection and results were accurately described. Consequently, reporting and attrition bias were considered at low risk. Finally, different techniques and technologies of VT ablation were utilised in the respective studies. In addition, differing procedural endpoints might have influenced the outcome and success in the three trials; these intrinsic technical aspects were judged to be at high risk of bias by both investigators. The risk of bias evaluation for all three studies is outlined in Supplementary Material Figure 2.
Discussion
The primary finding of our meta-analysis can be summarised as follows. Catheter ablation of VT after a first episode of VT in patients with ischaemic cardiomyopathy undergoing ICD implantation is effective in achieving a significant reduction in the occurrence of an appropriate ICD shock. Considering the mean rate of appropriate shock in the overall study population, an OR of 2.58 corresponds to an 84% increase in the relative risk of shock in the group that underwent ICD implantation without preventive VT ablation. Furthermore, patients without VT catheter ablation experienced a significantly higher risk of any appropriate ICD therapy compared with patients with VT ablation (OR 2.04; 95% CI [1.15–3.61]). With respect to the incidence of arrhythmic storm, a trend towards an increased risk was observed in the group without VT ablation without reaching statistical significance. Of note, there was no difference between the two approaches with respect to mortality. Regarding complications during short- and long-term follow up, no differences could be observed between the two groups.
Myocardial scarring associated with ischaemic heart disease acts as the underlying substrate for ventricular arrhythmias. The semi-vital and stunned myocytes scattered in between fibrosis or myocardial scarring generate an arrhythmic milieu for the re-entrant mechanism, enhanced automaticity and triggered activity, which can give rise to ventricular arrhythmias.18–20 It is well known that ICDs do not modify the underlying arrhythmic substrate, although effectively treating ventricular arrhythmias and preventing sudden cardiac death; in other words, it does not itself prevent the occurrence of arrhythmias. Of note, to date, there is no diagnostic test available that can reliably predict the occurrence of arrhythmias according to the characterisation of the underlying arrhythmogenic substrate. In fact, the onset of ventricular arrhythmias is unpredictable and not related to the size of the scar. For more than two decades, the ICD indication in patients with ischaemic heart disease has been based on the Multicentre Automatic Defibrillator Implantation Trial (MADIT) and Antiarrhythmics Versus Implantable Defibrillators (AVID) trial.21,22 As a result, a relevant number of patients with ischaemic heart disease worldwide are implanted with an ICD following the detection of an EF <30%. However, the long-term prognosis of these patients is afflicted by the sequence of ventricular arrhythmia recurrences, ICD shocks and increased mortality. In this scenario, the VTACH, SMASH-VT and SMS trials represent a major breakthrough.
All three trials tested the effectiveness and safety of an ablation strategy aiming to modify the underlying arrhythmic substrate in patients undergoing ICD implantation. Of note, the reduction of arrhythmic events achieved with this preventive ablation approach is relevant, as outlined by our analysis. In addition, preventive ablation did not result in a higher complication rate. Periprocedural complications may occur with VT ablation, particularly acute haemodynamic decompensation, given the multiple comorbidities usually found in patients with ischaemic cardiomyopathy and the haemodynamic effects of VT.23 Muser et al. developed a score to predict the risk of complications in patients undergoing ventricular tachycardia ablation. The main components of this score were: age, ischaemic cardiomyopathy, pulmonary disease, diabetes, New York Heart Association class, left ventricular EF and VT storm. Patients with VT have a higher burden of comorbidities, which increases procedural complications and mortality. The authors demonstrated that such risk may be mitigated by the use of mechanical support in selected cases.23
Recently, Atti et al. published another meta-analysis comparing the use of VT ablation before ICD implantation versus ICD implantation only, pooling data from the same studies.24 Despite showing similar results, we believe that our analytical approach in treating the OR and the direction of the comparison could be more helpful in streamlining the risks associated with the use of ICD implantation without the use of prophylactic VT ablation, and ultimately provide more clinically useful information in the decision-making process for the current daily practice.
Although this meta-analysis could show a significant reduction of ICD shocks and appropriate ICD therapies in patients undergoing preventive VT ablation, this did not result in a significantly lower mortality rate. Although there was a trend towards a lower mortality rate in the preventive ablation group, it did not reach statistical significance. Higher patient numbers and/or a longer follow-up duration are needed to evaluate the impact of VT ablation on mortality. In addition, novel ablation strategies, such as LAVA elimination, dechannelling, substrate isolation or scar homogenisation, might have resulted in a better outcome and a lower mortality rate.25–31
Indeed, there are unsolved questions (patient selection, timing of ablation, endpoints of the procedure), as noted by Mukherjee et al., for which current trial data does not provide enough evidence to advocate for prophylactic VT ablation.32
Future trials, such as the ongoing preventive aBlation of vEntricular tachycaRdia in patients with MyocardiaL INfarction (BERLIN VT; NCT02501005) trial, will evaluate whether preventive catheter ablation is superior to deferred catheter ablation.
Limitations
The main limitation of this analysis relates to the small number of patients randomised in these studies, which do not allow performing subanalyses of the groups at higher risk, such as patients with EF <30%. Another limitation is the short duration of follow up that may be inadequate to correctly assess hard endpoints, such as mortality. In addition, there is heterogeneity among the studies regarding the patients included, the type of intervention performed and the assessment of outcomes. Last, but not least, the low enrolment rates (e.g. <2 patients per year per institution in the SMS study) resulted in very long study duration and technological improvements with respect to ablation technologies and techniques over time that might influence outcome.
Conclusion
In patients with ischaemic heart disease and secondary prevention ICD implantation, preventive catheter ablation results in a significant reduction of appropriate ICD shocks and any appropriate ICD therapy compared with patients without or with deferred VT ablation. Furthermore, there was a trend towards an increased risk for VT storm in patients without VT ablation. No significant difference with respect to complications or mortality was observed between both treatment strategies.
Click here to view supplementary material.
Clinical Perspective
- Preventive VT ablation significantly reduces ICD shocks.
- Arrhythmic storm may be reduced by preventive VT ablation.
- Preventive VT ablation reduces all ICD therapy.
- Prophylactive VT ablation is not associated with significant procedural complications.