Catheter ablation has moved from ablation of ‘simple’ substrates like accessory pathways,1 atrioventricular nodal re-entrant tachycardias (AVNRTs)2 and re-entrant or focal tachycardia (of either ventricular or atrial origin)3–5 in recent years to more complex arrhythmias such as atrial or ventricular tachycardia (VT) or fibrillation.6–8 Even patients with complex congenital heart disease that may present with a very unusual cardiac anatomy are nowadays candidates for curatively intended catheter ablation procedures.9,10 Some patients are challenging because of the multitude of arrhythmias they present with (e.g. in Ebstein’s anomaly11 or patients after Fontan palliation12). This paper aims to review the contribution of advanced mapping technology using three-dimensional (3D) image integration and remote magnetic navigation (RMN) for patients with complex arrhythmia, or simple arrhythmias in the presence of congenital heart disease.
Remote Navigation
A detailed description of this system has been previously published.13,14 In brief, it consists of two computer-controlled permanent magnets positioned on either side of the fluoroscopy table (AXIOM Artis®, Siemens, Germany). A uniform magnetic field (0.08 or 0.10 Tesla) is created inside the chest of the patient of about 20 centimetres (cm) diameter. The soft mapping catheter aligns parallel to the externally controlled direction of the magnetic field due to three small permanent magnets embedded in the catheter tip. Changing the direction of the outer magnetic field navigates the tip of the catheter accordingly. The combination with a mechanical motor drive to advance or retract the catheter allows complete remote control of the catheter.14
Clinical Experience in Simple Arrhythmia Ablation
When using a novel system, it is wise to address simple procedures first and then progress to more and more challenging arrhythmias. This also allows the operator to convert to conventional ablation catheters should the remote procedure prove to be difficult or impossible. Clinical experiences from various groups around the world have been published over the last decade demonstrating the safety and effectiveness of the system in remote catheter ablation of supraventricular tachycardias (SVTs),14–19 right ventricular outflow tract tachycardia,20 atrial flutter21,22 and atrial tachycardias.23 Interestingly, right atrial isthmus-dependent flutter seemed to be a challenging arrhythmia, which was only successfully ‘solved’ when irrigated-tip catheters became available.23–25
Atrial Flutter Ablation with Remote Magnetic Navigation
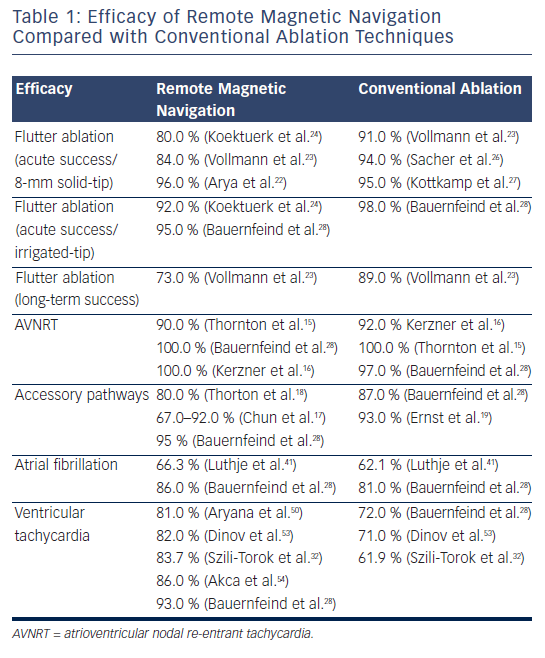
The acute ablation results for ablation of cavotricuspid isthmus-dependent flutter varied between 80 and 96 % when an 8-millimetres (mm) solid-tip magnetic catheter was used22–25 or 92 % when a 3.5-mm irrigated-tip magnetic catheter was used.24 Although the magnetic navigation approach led to comparable results acutely with lower fluoroscopy time, it required prolonged radiofrequency current application and procedure times compared with the conventional approach.26,27
Moreover, it appears that in the long term (six months) freedom from atrial flutter recurrence tends to decrease in the magnetic navigation group compared with the conventional approach (73 versus 89 %) in one prospective randomised study.23 Although inter-individual anatomical variations such as concave cavotricuspid isthmus, prominent pectinate muscles or existence of a sub-Eustachian pouches, can account for some of the failures,24 regardless of the techniques used,23 it has been suggested that the technology is more effective in creating focal effective lesions rather than deep linear transmural lesions, maybe related to the necessary design of the catheter, which requires the small magnets to be embedded in its tip.23 Additionally, the incidence of char formation with the 8-mm solid-tip catheter seemed to have been higher, possibly due to a reduced tip-to-tissue surface area of contact.
Solutions to overcome these potential difficulties include looping of the catheter with placement of the tip of the ablation catheter more parallel to the tissue or in a more wedged position, delivering more power for longer duration, increasing the strength of the magnetic field in order to allow for better contact and higher energy delivery or, more importantly, using irrigated-tip catheters (see Table 1).24,25,28
Remote Magnetic Navigation in Simpler Electrophysiological Substrates
As mentioned above, the results of some of the previous studies did not support the use of the RMN system with an 8-mm tip electrode for ablation of common right atrial flutter.23 However, encouraging acute results are reported when the 3.5-mm irrigated-tip catheter was utilised (see Table 1).24,25,28
Atrioventricular Nodal Re-entrant Tachycardia
Previous studies have emphasised the efficacy and safety of the technique in treating more discrete substrates such as slow pathway ablation/modulation for atrioventricular (AV) re-entrant tachycardia.15,16 When direct comparison was made between RMN ablation and conventional approach, magnetic guidance seemed similar, if not superior, in terms of efficacy compared with conventional catheter ablation of AVNRT. Previous studies reported comparable results when conventional techniques were used.29–31 Although a longer time between insertion of the ablation catheter and placement of the first radiofrequency lesion was necessary in the magnetic-guided ablation cohort; there was a trend towards a shorter total radiofrequency time in the magnetic guidance group.16 Moreover, the median physician radiation exposure time was three-times lower than the patient exposure time.15 No significant complications occurred. One transitory AV block reported in the magnetic group fully recovered.16
Accessory Pathways
In patients with other discrete substrates, such as accessory pathways, remote magnetic-guided ablation showed initially moderately good results,17,18 but outcomes improved dramatically with the learning curve and perfected technology,17,28 with safe profile demonstrated even in the most challenging situations such as para-Hisian substrates (see Table 1).19
Idiopathic Ventricular Tachycardia
Idiopathic ventricular arrhythmias also seem to be safely and effectively targeted with the RMN system. The magnetic-guided mapping and ablation appear particularly useful in difficult positions, such as aortic cusps or papillary muscle VTs.28 In these situations, manual navigation is significantly restricted by the multiple curves of the catheter, whereas the manoeuvrability of the soft magnetic catheter is not hindered by the specific location. Moreover, the stability of the magnetic catheter due to the constant magnetic force directing the tip during the radiofrequency application makes the procedure safer and more effective.20,28,32
Remote Magnetic Navigation for Atrial Fibrillation Ablation
After the favourable outcomes of the SVT ablation procedures, the next ‘goal’ was naturally to perform remote-controlled atrial fibrillation (AF) procedures.33 However, an 8-mm ablation catheter was the only alternative available to the 4-mm tip catheter used in SVT ablations; it was to no surprise that remote ablation was possible, but ran the known higher risk of clot formation at the tip (as compared with irrigated-tip catheters).34 Although the initially reported results in AF ablation using the RMN system and solid-tip magnetic catheter varied significantly among groups33–41 (see Table 1); more recent data using irrigated-tip magnetically navigated catheters showed more promising results with increased safety profile. The introduction of a magnetic catheter with an irrigated-tip allowed avoidance of the thromboembolic risk and several groups have published their results, which are comparable to conventional technologies.35–41 Two centres have jointly published their experience in a total of 71 patients with either paroxysmal or persistent AF. They reported safe and effective remote-controlled manipulation for reconstruction of the left atrium (LA) and mapping of the pulmonary veins (PVs). In three patients, a cross-over to conventional manual PV mapping was necessary to position the catheter in the right inferior PV.42
Additionally, the use of RMN was associated with markedly reduced fluoroscopy time, although at the expense of prolonged ablation and procedure durations. The lower fluoroscopy time likely results from the greater flexibility of the soft tip of the catheter, which does not require frequent radioscopic visualisation. Additionally, the risk of cardiac perforation remains extremely low in most studies. Although it has been argued that the operator must commute frequently between the control room and the operating room, the overall physical fatigue for the operator is markedly reduced when the system is used, as is the fluoroscopy exposure. Moreover, an additional mechanical ‘V drive’ has been recently introduced that allows to remotely steer a circumferential PV mapping catheter as well42 and avoid commuting (see Table 2).
Combination of 3D Electroanatomical Mapping and Remote Magnetic Navigation
Using the integrated 3D electroanatomical system CARTO® RMT (Biosense Webster, Brussels, Belgium), the different vectors needed for 3D mapping are applied from the mapping system and the 3D reconstruction is displayed on the navigation workstation.37,43 All three systems (magnetic navigation, 3D mapping system and fluoroscopy) are registered such that the information is shown in a single combined fashion with picture-in-picture display. This was of great advantage during complex ablation procedures and allowed further reduction of the overall fluoroscopy exposure.
Additionally, both realtime and snapshot reviews of the intracavitary tracings can be displayed on the same screen. All the above generally imply less physical strain for the operator, and potentially better operator concentration, and more detailed and careful electrogram analysis. With the advent of the 3.5-mm atraumatic irrigated-tip magnetic catheters that can be used within the CARTO RMT platform, the risk of perforation, thromboembolic events and char formation have also decreased considerably.
Remote Magnetic Navigation for Ventricular Tachycardia Ablation
After the initial reports on right VT ablation,20,44 which in itself is a rather simple target in an easily accessible area, more difficult substrates of VT (including ischaemic and dilative cardiomyopathy) were targeted.32,45–50 Parallel to the growing evidence of conventional epicardial ablation for VT, the magnetic navigation system has been used in the epicardial space, which became possible when the irrigated catheter became available.50,51
Results on safety and feasibility of the technology in diagnosing and treating complex ventricular arrhythmias were initially reported in 200750,52 in both animal models52 and in humans,50 with good overall results and minimal fluoroscopy exposure using a 4-mm solid-tip magnetic catheter. Dinov et al.53 retrospectively analysed the long-term efficacy of a single procedure ablation for ischaemic VT and compared it with manual radiofrequency ablation in 102 patients using irrigated-tip catheters. Magnetic-guided radiofrequency ablation of ischaemic sustained VT proved to be equally efficient compared with manual ablation in terms of acute and long-term success rate, with the additional benefit of a significantly reduced fluoroscopy time and shorter radiofrequency time.
Similarly, Bauernfeind et al.28 have reported their overall experience with the RMN system in 610 patients, 83 of whom had undergone ablation for VT. The superiority of the magnetic system became more prominent in the VT group compared with other types of tachycardia where results were roughly comparable. More specifically, in the idiopathic forms of VT, where the substrate is more discrete, and stability and precision of the radiofrequency delivery is key, the system proved more efficacious than in the rest of the VT group – in the magnetic navigation group the success rate was 97 % compared with 79 % in the conventional group (p=0.026). Specific features of the system such as manoeuvrability, stability and constant contact seem to be particularly useful in these situations.
Although randomised data are scarce, a recent systematic review54 of the experience with the remote navigation system in VT ablation across 11 studies in 110 patients showed similar results, with an overall need for crossover to manual ablation in 14 % of the cases. Six patients out of the 110 developed complications: one AV block, two groin haematomas, one deep vein thrombosis, one stroke when a solid-tip magnetic catheter was used and a right ulnar palsy. Overall, a higher acute success rate (97 versus 81 %, p=0.03) and lower rate of arrhythmia recurrence (14 versus 50 %, p<0.01) were achieved in magnetic navigation procedures compared with manual catheter ablation. The use of the RMN system reduced the occurrence of major complications (0.34 versus 3.20 %, p=0.01) without compromising the efficacy compared with manual ablation. Additional decreased time of fluoroscopy exposure was noted across most studies.54
Remote Magnetic Navigation for Ablation of Patients with Congenital Heart Disease
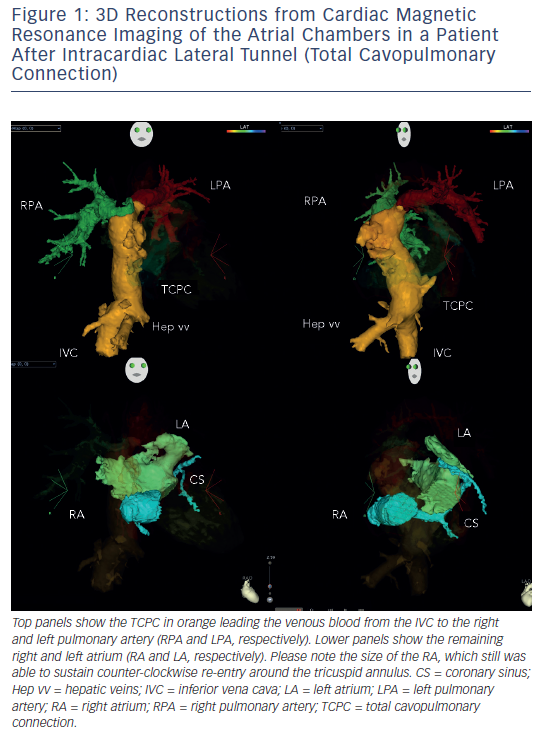
Arrhythmias in patients with complex congenital disease are a source of significant morbidity, haemodynamic deterioration and mortality.9,10 Medical treatment proves to be of limited efficacy, and ablative procedures have emerged as an alternative, but long-term results after ablation are somewhat sobering. In order to perform an ablation procedure in this patient cohort successfully, a careful review of the individual anatomy and details of the performed surgery (if applicable) needs to be performed. Precise understanding of the underlying anatomy (e.g. presence of intra-atrial baffles or baffle leaks) and the location of the conduction system is necessary to plan and execute an ablation procedure. Pre-procedural imaging and 3D reconstruction is of particular importance, since it is demonstrating the ‘up-to-date’ anatomical situation (see Figure 1). This can be assessed either by computerised tomography scans or by cardiovascular magnetic resonance imaging. Retrograde transaortic approach using magnetic navigation is particularly useful in patients with congenital heart disease and “difficult access” to the targeted chamber.
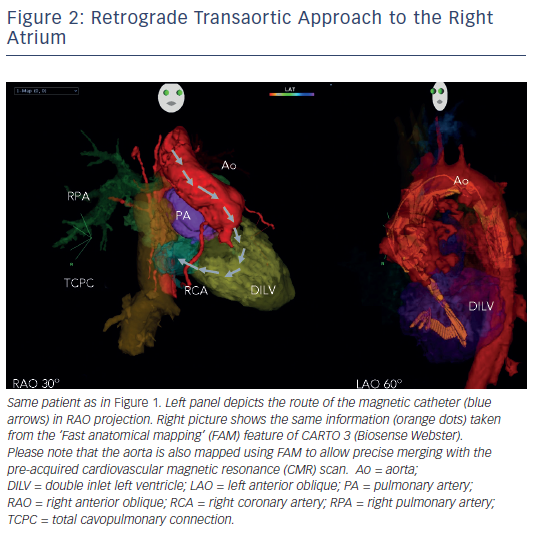
In patients with transposition of the great arteries (TGA) after Mustard/ Senning operation, magnetic navigation is especially convenient by using a retrograde approach to cross the aortic valve (see Figure 2). Similarly to the experience in left-sided accessory pathway ablation, the magnetic catheter can then be navigated across the AV valve to sequentially map the atrial chambers during permanent tachycardia. Several groups have confirmed the applicability of the remote navigation system in this patient cohort, with excellent outcome data.55–57
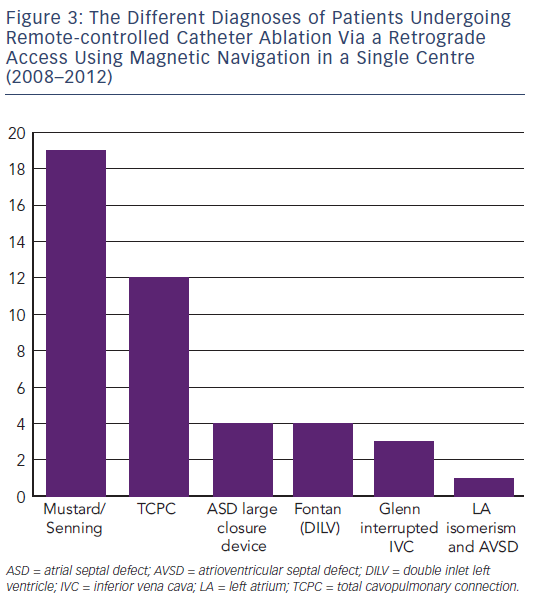
Another challenging group consists of patients with physiologically univentricular hearts that underwent surgery creating either an atriopulmonary (AP) Fontan (right atrium [RA] to pulmonary artery)58 or its modern modification of total cavopulmonary connection (TCPC)59 (see Figure 3). The prevalence of post-Fontan patients with SVTs is continuously increasing as the population ages and is associated not only with morbidity but also mortality.60 Although numerous studies have been published on mapping and ablation of univentricular heart-associated SVTs,12,61–64 most of them focused on AP-Fontan and relatively little is known about TCPC-associated SVTs. In the TCPC cohort, access to the tunnel can be easily achieved by venous puncture, but the atrial chambers are not directly accessible. Using the same retrograde approach as in the transposition of the great arteries (TGA) group, retrograde access was easily achieved and allowed mapping of both atrial chambers (see Figure 2). We recently demonstrated that TCPC patients in fact present with various arrhythmias, which consist mostly of re-entry around the right-sided AV valve (50.0 %), but also with ectopic tachycardia (12.5 %) and AVNRT (37.5 %).65 All arrhythmias were addressed using the RMN system in combination with 3D electroanatomical mapping and 3D image integration. Despite the need for a mean of 1.5 procedures (mostly driven by redo procedures in one of the AVNRT cases), clinical follow-up so far has been favourable.
In the Fontan cohort, magnetic navigation does not intuitively seem to contribute beyond the advantage of 3D image integration and reach of the catheter. Large curve conventional catheters might still be difficult to manipulate in these massively enlarged RA; the advantage of higher contact force may allow delivering of full thickness lesions better than the magnetic system. However, especially in patients with double inlet ventricles who have undergone patch closure of an AV valve, magnetic navigation assists in gaining access to the space behind the patch.66 This is the missing part in peri-AV valve re-entry and access is gained retrogradely, followed by irrigated-tip catheter ablation. This might in fact be one of the explanations why double inlet left ventricle (DILV)-Fontan patients had a higher recurrence rate of atrial flutter since the so-called isthmus line was never blocked when ablating conventionally.9,12
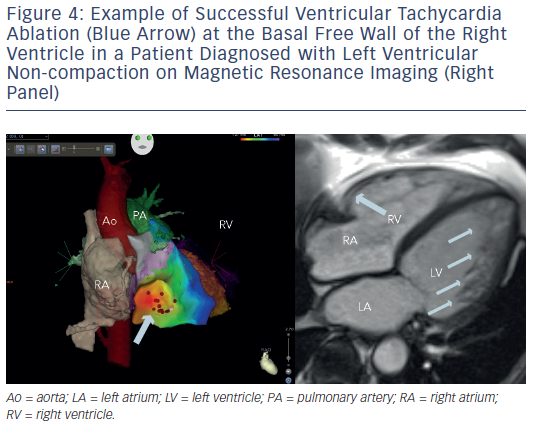
Ventricular tachycardia is another challenge in the setting of adult congenital heart disease, since these patients are at a significantly higher risk of sudden cardiac death than their peers with normal cardiac anatomy. Patients with systemic right ventricles or after ventricular repair (e.g. in tetralogy of Fallot) are at particular risk.66–70Figure 4 gives an example of VT ablation in left ventricular non-compaction. In the majority of patients, epicardial access will not be an alternative since post-operatively most patients will no longer have a true epicardial space. Again, remote navigation will find its role in this cohort whenever endocardial access to a given area is challenging.
Conclusion
Especially when addressing catheter ablation in arrhythmias in complex patients (e.g. after reparative surgery for congenital heart disease), RMN is a valuable alternative to conventional ablation tools. The combination of magnetic navigation with 3D electroanatomical mapping systems and 3D image integration as well as the availability of irrigated tip equipped magnetic catheters were key for this achievement.