Disputation remains the lifeblood of science. However, when seeking to adjudicate disputes, it is always wise to take note of previous debates. In this regard, the anatomy of the atrioventricular conduction axis has proved to be a hotbed of disagreements since its initial descriptions.1 The topic has now achieved increased importance for those seeking specifically to pace the axis. In a recent review, for example, Vijayaraman et al. emphasised the importance of understanding its anatomical location.2 Vijayaraman et al. gave appropriate credit in this regard to the initial accounts provided by His and Tawara, with the latter masterpiece now available in an English translation.3,4 Vijayaraman et al. correctly stressed the relationship of the atrioventricular conduction axis to the membranous septum, yet then illustrated the left bundle, as seen from the left side, as descending from the left atrium at the midpoint of the orifice of the mitral valve.2 They cited the work of McAlpine as underscoring their depiction. However, in his Atlas, McAlpine shows the left bundle emerging from the inferoseptal recess, although he does not specifically name the recess.5 The dependency on the work of McAlpine is then also evident in another recently produced diagram, with the purpose of illustrating the potential sites of block in the left bundle.6 Surprisingly, however, the arrangement is shown using a drawing of the parietal wall of the left ventricle, despite the bundle branches being supported by the ventricular septum. The authors of that review then depict the right bundle branch as turning back into the left atrioventricular groove.6 Such diagrams are unlikely to provide the anatomical precision rightly demanded.2,6 In a more recent article, dissections prepared by ourselves have been used to provide the necessary information.7 However, our own knowledge has advanced since we produced those dissections.8
In this review, therefore, we offer our latest understanding of the location and relationships of the atrioventricular conduction axis. We include details of its development and emphasise the different arrangements to be found in the human heart compared with dogs or oxen.9,10 In particular, we emphasise the importance of understanding the relationship between the inferior pyramidal space and the inferoseptal space, parts of the heart that, thus far, have received little attention.11 The anatomical detail needs to be placed in the context of the heart as seen within the body; that is, using attitudinally appropriate nomenclature.12 We point, however, to the similarity to be found between the arrangements as found in the human and murine hearts.13 Another aspect then needs to be fulfilled if all this information is to meet the expectations of those promoting the significance of pacing the ventricular components of the axis.2,6,7 The anatomical detail needs to be placed in the context of the heart as seen within the body, in other words, using attitudinally appropriate nomenclature.13
Displaying the Anatomy in an Attitudinally Appropriate Fashion
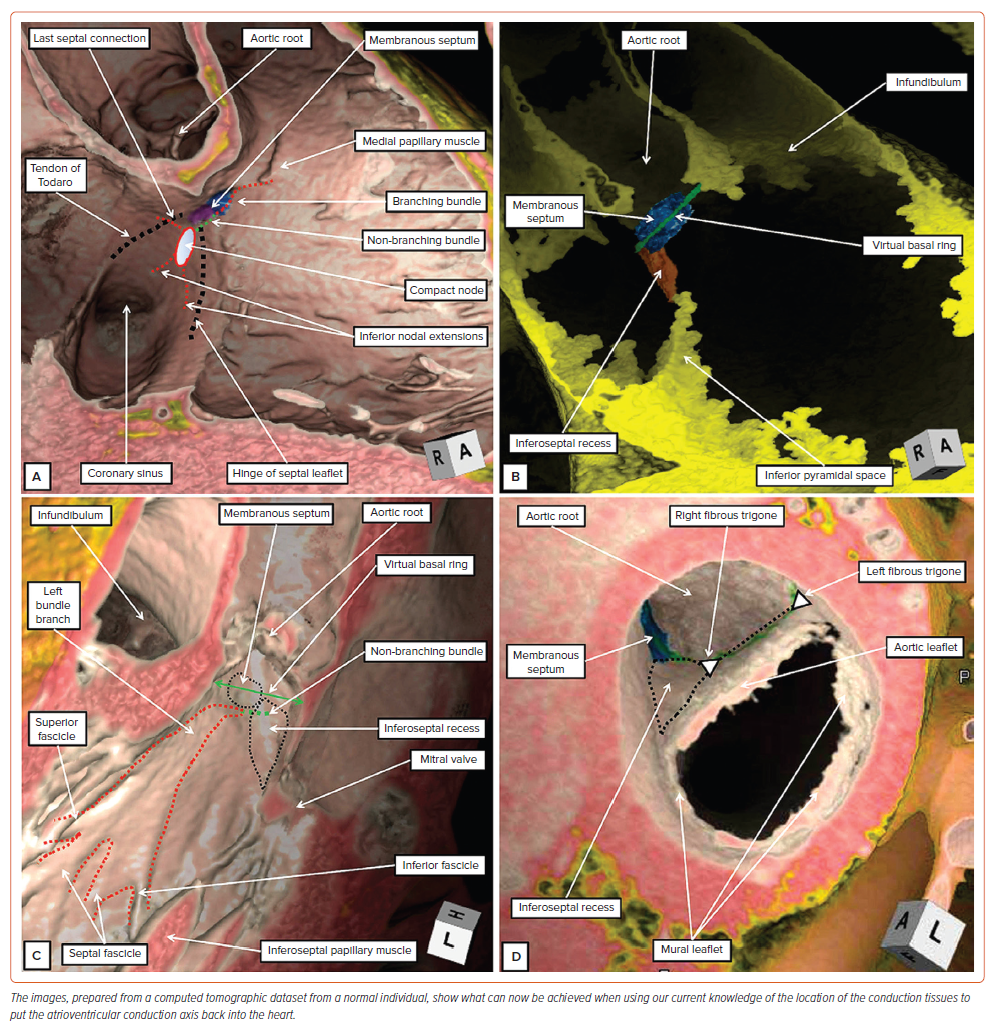
It is in the field of electrocardiography, which continues to play a major role in underscoring the diagnoses made by electrophysiologists, that an attitudinal approach to nomenclature becomes most important. This, of course, is because the ECG is recorded on the basis of the patient standing upright and facing the observer. It is also the first rule of cardiac anatomy that all structures should be described relative to the coordinates of this anatomical position.12 It remains a mystery as to why, for centuries, anatomists have ignored this rule when describing the cardiac components. There are now no excuses for cardiologists not to apply the rule because, increasingly, the images produced by their diagnostic techniques show the heart as it is positioned within the body. When using CT datasets, for example, it is now an easy matter to produce virtual dissections showing the arrangement of the cardiac components relative to the bodily coordinates (Figure 1). Based on our current knowledge of the location of the components of the atrioventricular conduction axis, this being derived from histological examination of a large series of serially sectioned datasets from human hearts, we are now able to provide accurate indications of their positions within the hearts as seen attitudinally (Figures 1–3).14–17
The virtual dissections showing the septal aspect of the right-sided chambers reveal the landmarks of the triangle of Koch, and the site of the medial papillary muscle (Figure 1A). This information provides the necessary landmarks to place the axis within the heart. When viewed in attitudinally appropriate fashion, the apex of the triangle is located superiorly relative to its base, not to the right as it was often depicted in the past. The atrial components of the axis are found within the apex of the triangle.14 The right bundle branch then emerges in the right ventricle beneath the base of the medial papillary muscle.15 Joining together the two landmarks shows the anticipated site of the ventricular component of the conduction axis (Figures 1A and 3B). More extensive virtual dissections show the fat filling the inferior pyramidal space as an extension from the inferior atrioventricular groove. The relationships within the heart can only be properly understood when these features are assessed using the attitudinal approach. This reveals how the superior apex of the space is adjacent to the inferior extent of the inferoseptal recess (Figure 1B). It is this relationship that is key to appreciating the transition of the axis from its atrial to its ventricular components. The dissection also shows how it is possible to construct the virtual basal ring of the aortic root, and to demonstrate the relationship of this ring to the membranous septum (Figure 1B).
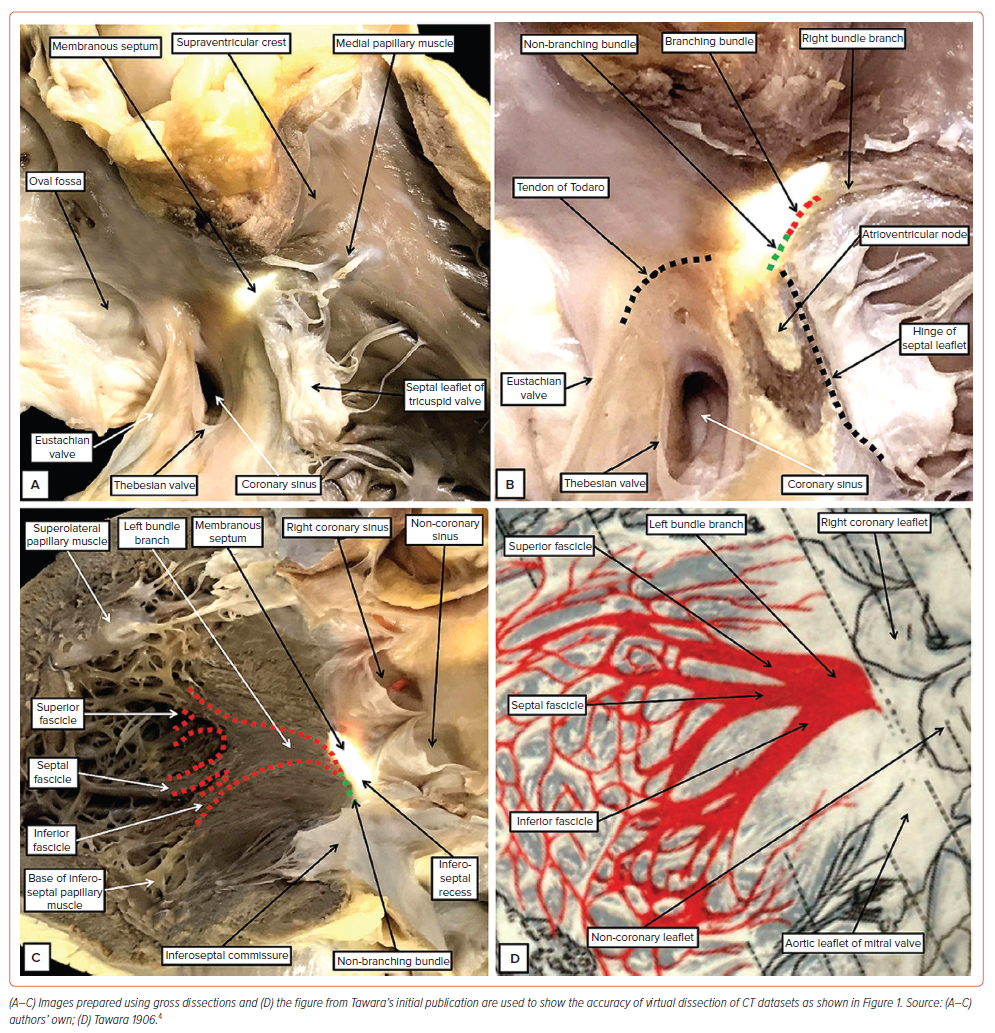
Attention to the left side then clarifies the relationships between the membranous septum and the aortic root (Figure 1C). The fibrous part of the septum is part of the so-called ‘central fibrous body’. This extensive area of fibrous tissue, in addition to the membranous septum, is made up of an area of fibrous continuity between the leaflets of the mitral and tricuspid valves, along with the right fibrous trigone. The trigone is the thickened rightward extent of the area of continuity between the leaflets of the aortic and mitral valves (Figure 1D and Supplementary Figure 1). The left fibrous trigone, which is the thickened leftward end of the area of fibrous continuity, anchors the aortic–mitral unit to the parietal wall of the left ventricle (Figure 1D). Comparisons of the virtual dissections with gross dissection of an autopsied heart (Figure 2) demonstrate their accuracy in identifying the key landmarks required to predict the location of the conduction axis.15 Important details are then provided when the appearance of the left bundle branch, as seen in a gross specimen (Figure 2C), is compared with the drawing provided by Tawara to demonstrate its location in the human heart (Figure 2D).4 The images show that the fascicles of the left bundle are found in superior, septal and inferior positions, rather than being positioned ‘anteriorly’ and ‘posteriorly’. This fact again points to the need for an attitudinal approach. Our own histological findings have served to confirm the initial description provided by Tawara, as did the investigations of Demoulin and Kulbertus, and Massing and James.4,18,19 All investigations showed the left bundle to ramify in trifascicular fashion. There is no evidence to support the notion that the left bundle is bifascicular, a concept still promoted by some investigators.20
Histological Definition of the Components of the Atrioventricular Conduction Axis
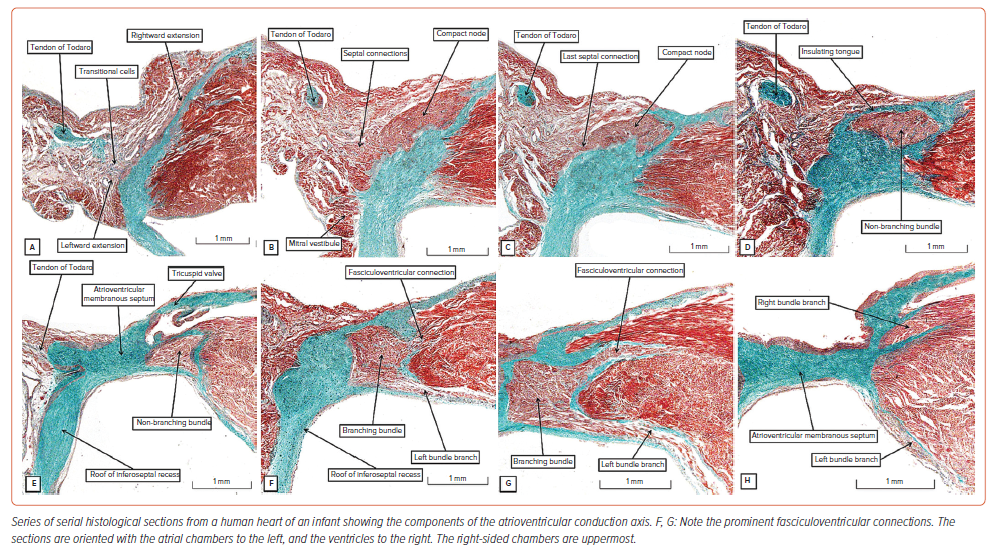
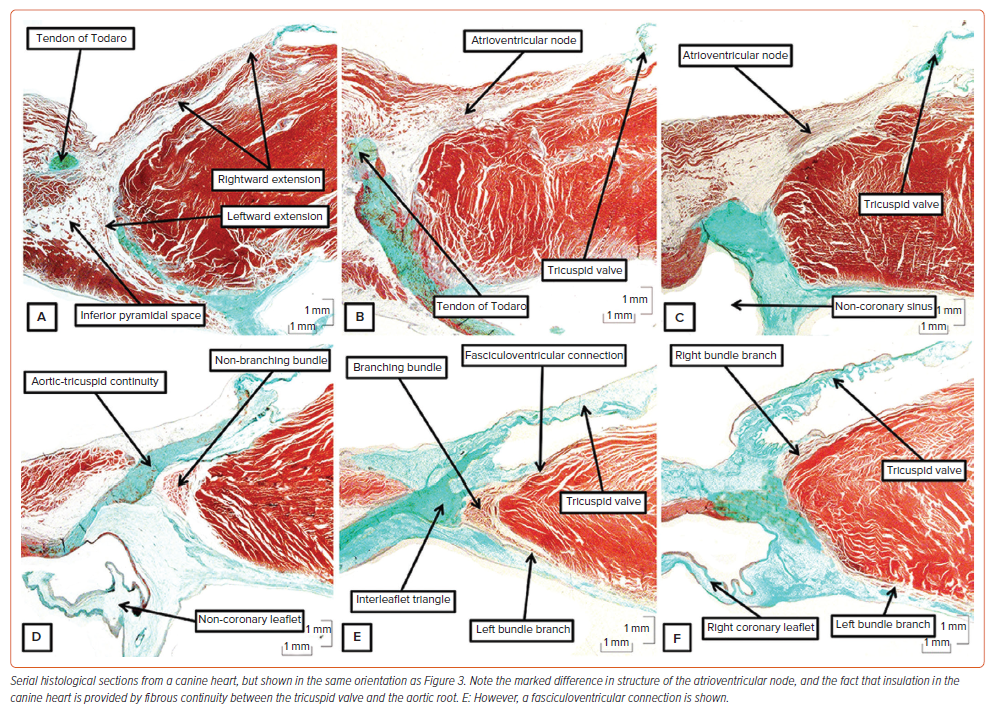
It is difficult, even now, to improve on the initial account provided by Tawara regarding the make-up of the atrioventricular conduction axis.4 As he explained, the axis takes its atrial origin from the compact node, which is found within the confines of the triangle we now name for Koch.16 The histologically specialised inputs to the node can be recognised as extensions running within the vestibules of the mitral and tricuspid valves (Figure 3A).14 The vestibules themselves form the right and left walls of the inferior pyramidal space.11 When traced superiorly, the inferior nodal extensions merge to form the body of the atrioventricular node. Having established its body from the vestibular components, the node then receives additional inputs from the buttress of the atrial septum (Figure 3B).14,16 As the axis is traced towards the apex of the triangle of Koch, the histologically specialised myocardium becomes separated from the working atrial cardiomyocytes by a tongue of fibrous tissue, with the tongue itself producing continuity between the leaflets of the mitral and tricuspid valves (Figure 3D). There are no obvious histological differences between the axis before and after it becomes insulated from the atrial myocardium. This point is of significance with regard to the issue of longitudinal dissociation within the axis. Much is made of the importance of such separation providing pathways through the axis that are predestined to become the left as opposed to the right bundle branches. Those arguing in favour of such longitudinal dissociation cite the investigation of James and Sherf.21 These investigators, however, acknowledged the presence of lateral connections between the cardiomyocytes making up the axis. Furthermore, their description of multiple cells types within the non-branching bundle does not accord with our own observations. And, when we specifically examined the axis in human, canine and bovine hearts, we were unable to find evidence supporting the concept of longitudinal dissociation.22 In this regard, of equal significance is the location of the lesions producing bundle branch block. The proponents of longitudinal dissociation argue that this feature points to the presence of a lesion within the non-branching component of the axis as producing the block. It is true that, in the setting of ischaemic heart disease, isolated lesions can be found within the non-branching bundle. However, such lesions are found along with much more widespread lesions involving the fascicles of the left bundle as they descend the ventricular septum.23–25 The entirety of the left bundle is typically involved in the disease process.
The last connection between the atrial septal myocardium and the axis prior to its insulation (Figure 3C) likely functions as the fast pathway into the node, with the rightward inferior extension representing the slow pathway (Figure 3A).16 Having been insulated from the atrial septal myocardium, the ventricular component of the axis extends for a variable distance as the non-branching bundle. When we measured the extent of this non-branching component in a series of 21 adult human hearts, we found it varied in length from 1.7 to 7.2 mm, with a mean length of 3.6 mm.26 This non-branching part of the axis is located within the right fibrous wall of the inferoseptal recess, which becomes confluent with the atrioventricular component of the membranous septum. It is usually covered on its right side by an overlying area of the right atrial vestibular myocardium (Figure 3D). The non-branching component can then usually be traced towards the location of the hinge of the septal leaflet of the tricuspid valve. This hinge divides the membranous septum into its atrioventricular and interventricular portions. Having crossed the site of the hinge, the conduction axis is most frequently sandwiched between the ventricular component of the membranous septum and the crest of the muscular ventricular septum (Figure 3E). The axis in this location has usually ceased to be non-branching as it gives rise to the fascicles of the left bundle (Figure 3F). As we have already emphasised, and as shown by Tawara (Figure 2D),4 the fascicles extend into the left ventricle in subendocardial fashion in inferior, septal and superior directions. The inferior and superior fascicles can then be traced towards the inferoseptal and superolateral papillary muscles of the mitral valve. However, Tawara made no mention of the presence of direct connections between the insulated ventricular components of the axis and the crest of the muscular ventricular septum (Figures 3F, G). These fasciculoventricular connections were first recognised by Mahaim.27 Hecht et al. described them as the superior septal pathways when reviewing the arrangement of the axis.28 Furthermore, James and Sherf noted their presence when suggesting the presence of longitudinal dissociation within the axis, but made no mention of their potential significance.21 Lev and Thaemert noted their presence in the murine heart.13 Furthermore, any significance in normal conduction has received little subsequent attention.29 As we have now shown, careful study of serial histological sections confirms their presence in the majority of normal hearts.21 As we discuss below, the pathways could well activate the ventricular myocardium during selective pacing of the conduction system.30
Having given rise to the fascicles of the left bundle from the part of the axis sandwiched between the interventricular membranous septum and the crest of the muscular septum, the axis then forms the cord-like right bundle branch (Figure 3H). Usually subendocardial, it can also be intramyocardial. It is usually found within the right ventricle at the base of the medial papillary muscle (Figure 2B). It then continues within the substance of the septomarginal trabeculation, or septal band, ramifying into the ventricular apex through the moderator band. Branches from the bundle can also be traced superiorly into the subpulmonary infundibulum.31
The histological images shown in Figure 3 were prepared using a neonatal human heart. Transitional cells can often be identified in neonatal and infant hearts between the atrial cardiomyocytes and the histologically specialised cardiomyocytes of the atrial components of the axis (Figure 3A). Such transitional cells are also seen in foetal datasets, which additionally reveal the presence of nodoventricular connections (Supplementary Material Figure 1). It is unusual to find transitional cells in datasets from adults. The basic arrangement, nonetheless, is maintained through postnatal life, along with the presence of fasciculoventricular connections. However, the nodoventricular pathways seen in foetal hearts and the septal pathways arising from the non-branching bundle are not found in postnatal hearts. There is also variation to be found in adult hearts regarding the relationship between the non-branching and branching bundles and the crest of the muscular ventricular septum, as was emphasised by Kawashima and Sasaki.32 However, the relationship of the membranous septum itself also shows marked variation. We found marked differences in the component of the septum, as judged by transillumination, to be located within the aortic root, defining the virtual basal plane as the proximal extent of the root.17 As had been predicted by Keith and Flack, we now consider this variation to reflect the extent of outflow myocardium that supports the leaflets of the aortic valve.33 We show an example in Supplementary Material Figure 2, in which a significant segment of outflow myocardium interposes between the membranous septum and the site of entry of the axis into the inferoseptal recess. Furthermore, the axis does not always terminate having given rise to the right bundle. In a proportion of hearts, the axis can be traced into the aortic root as the so-called ‘dead-end tract’.34,35 As we describe below, this is well explained on the basis of knowledge of its development.
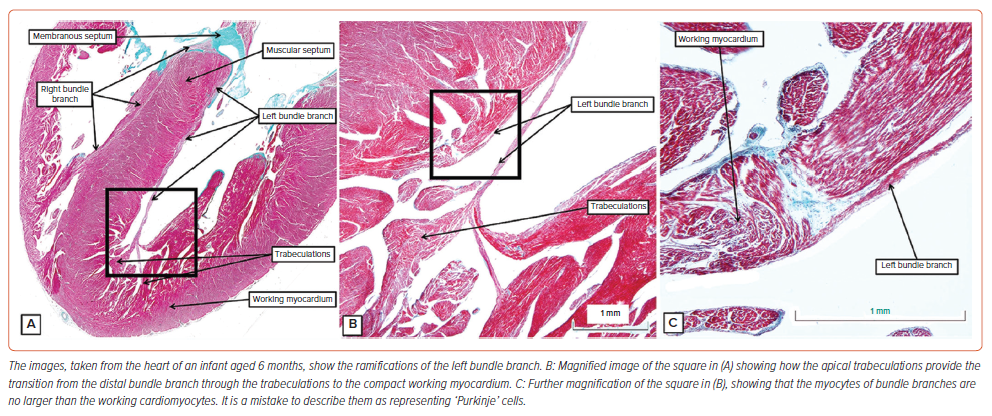
As the bundle branches descend along the septum towards the ventricular apexes, they are insulated from the working cardiomyocytes (Figure 3H). The specialised cardiomyocytes themselves are comparable in size to the adjacent working cells (Figure 4). It is a mistake to describe them as ‘Purkinje’s cells’. The cardiomyocytes, as described by Purkinje, are to be found in bovine hearts (see below). In these species, as shown by others, they can be traced into the ventricular walls.36,37 This is not possible in the human heart. Having lost their insulating sheaths, the specialised cardiomyocytes merge with the apical trabeculations, which, in turn, merge with the working cardiomyocytes of the ventricular walls (Figure 4A, B). In the human heart, it is not possible to trace the cardiomyocytes of the bundle branches into the compact wall as alleged ‘Purkinje’ cardiomyocytes.
The Superior Septal Pathways
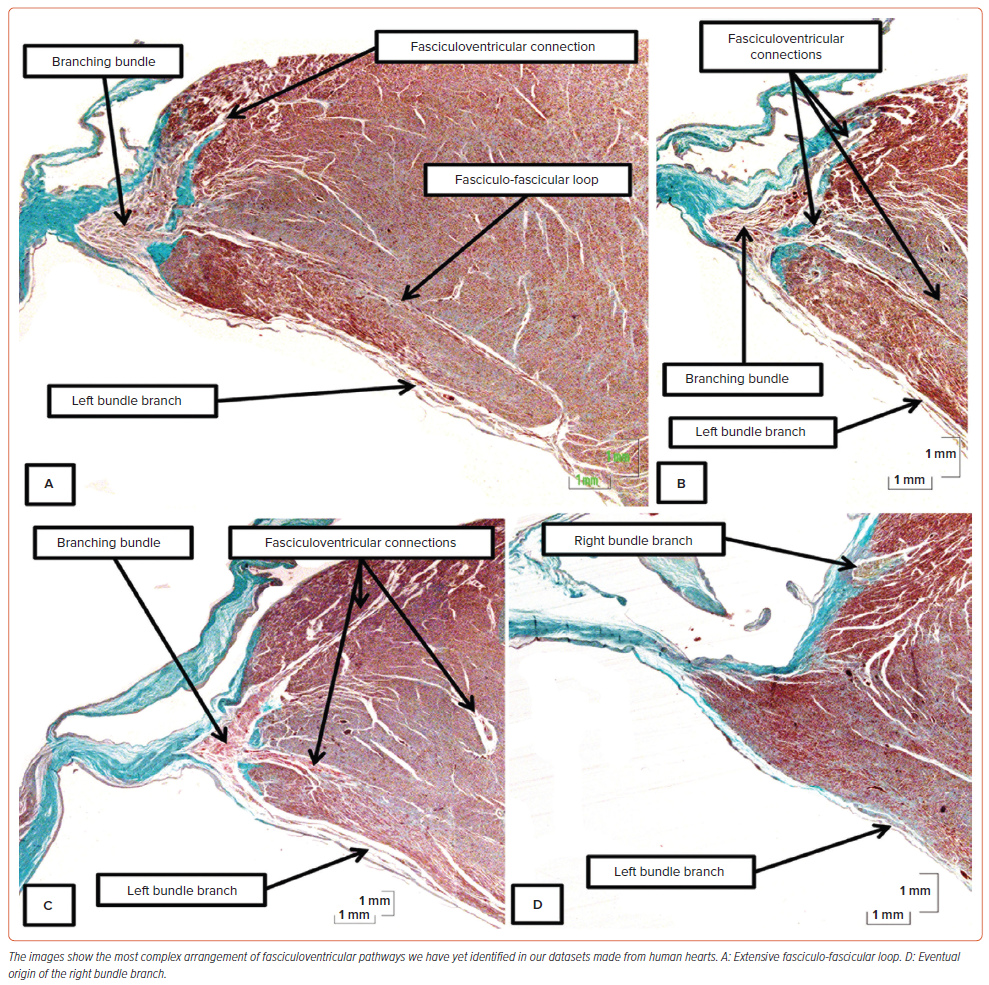
The connections between the ventricular components of the conduction axis and the crest of the muscular ventricular septum were initially described by Mahaim as a ‘paraspecific’ system for conduction.27 As we have already indicated, the pathways were included as ‘superior septal pathways’ by Hecht et al. when they summarised the overall arrangement of the axis.28 We had emphasised their potential role, along with nodoventricular connections, as a substrate for ventricular pre-excitation.38 We had ignored their potential role in activation of the ventricular walls in the absence of pre-excitation. In our earlier investigations, we had also failed to note their potential ubiquity, as had been described by Mahaim for the human heart, and by Lev and Thaemert in the heart of the mouse.13,27 On conducting a detailed examination of the non-branching and branching components of the axis in 21 human hearts, we identified the pathways in 14 datasets.26 In all these hearts, the pathways took their origin from the branching component of the axis. In three of the hearts, the pathways formed loops with the cardiomyocytes of the left bundle, with one of these loops being extensive (Figure 5A). In the heart with the extensive loop, additional pathways merged with the right side of the septum before the right bundle branch emerged in its anticipated cord-like fashion (Figures 5B–D).
Development of the Atrioventricular Conduction Axis
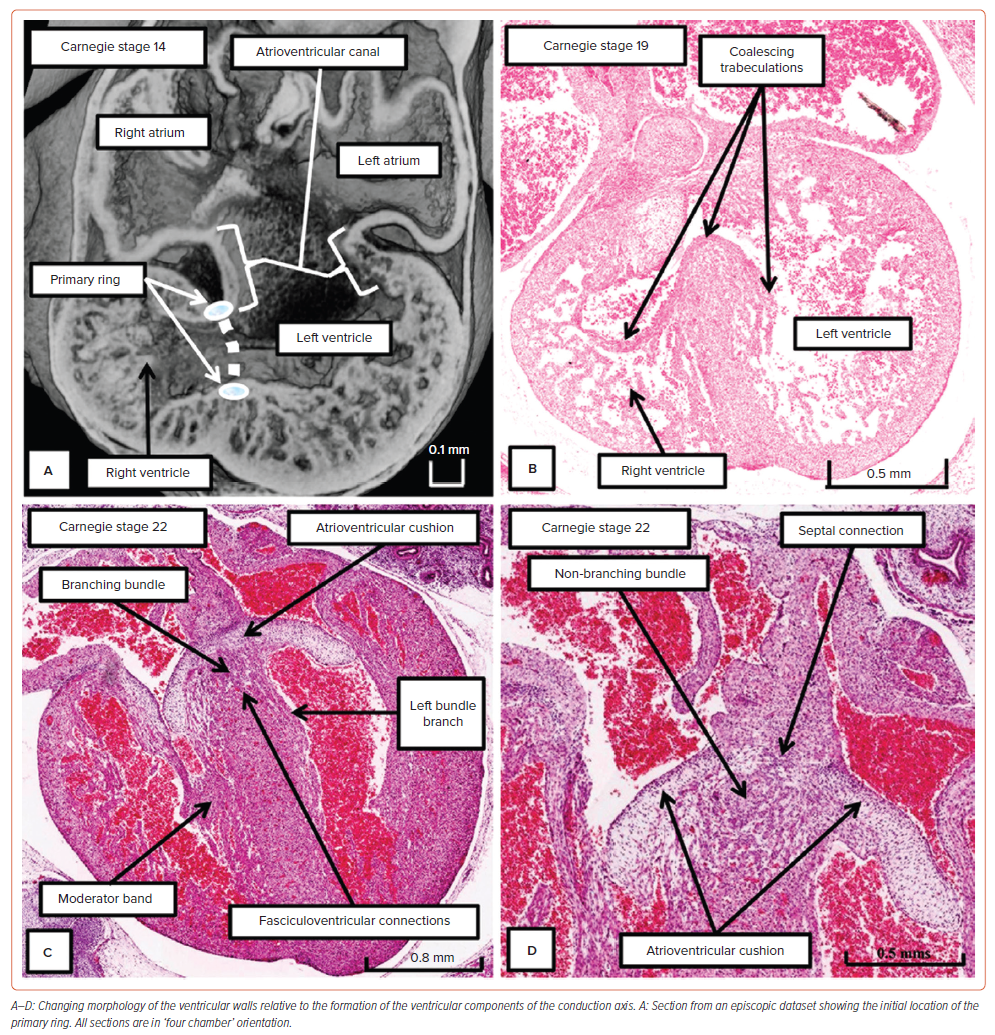
Our knowledge of development now permits us to provide explanations as to why the conduction axis becomes insulated by the tongue of fibrous tissue formed between the leaflets of the mitral and tricuspid valves, and why the extension of the axis is to be found, in some hearts, as a dead-end tract. The developmental findings also provide an explanation for the existence of the nodoventricular and fasciculoventricular pathways.9 In the early stages of development, subsequent to looping of the heart tube, the atrioventricular canal is supported exclusively by the developing left ventricle, whereas the outflow tract arises exclusively above the cavity of the developing right ventricle. Already at this early stage, it is known that the heart is able to induce atrioventricular delay. This is produced by the atrioventricular canal myocardium.39 However, the right side of the atrioventricular canal is contiguous with the cranial margin of the interventricular communication (Figure 6A). Using an antibody to the nodose ganglion of the chick, it proved possible to identify a ring of cardiomyocytes encircling the foramen.40 By tracing the fate of the identified cardiomyocytes, it was shown that the part of the ring carried on the crest of the developing septum was destined to become the ventricular component of the conduction axis.37 With expansion of the atrioventricular canal to provide the right ventricle with its own inlet, the part of the ring initially contained within the margin of the foramen contiguous with the canal becomes incorporated in the vestibule of the tricuspid valve.9 There are then superior and inferior transitions between the component carried on the crest of the ventricular septum and the part incorporated into the vestibule of the tricuspid valve. The inferior transition will become the atrioventricular node and its rightward inferior extension. The superior transition is sequestrated as the so-called ‘retroaortic node’. The part of the axis extending from the crest of the septum to the site of the retroaortic node attenuates almost completely. It is the remnants of this part of the initial ring that persist in some hearts as the dead-end tract.34,35
During these stages, the ventricular septum itself is in the process of being formed by coalescence of trabeculations. In these early stages of development, the trabecular myocardium makes up the greater parts of the thickness of the parietal ventricular walls (Figure 6A). With ongoing development, parts of these trabeculations condense to form the right and left bundle branches (Figure 6B). It is often thought that the trabeculations also ‘compact’ to produce the ventricular walls. There is no evidence to support this notion.41 Not all the trabeculations become insulated to form the bundle branches. Nonetheless, the trabecular myocardium does coalesce to form, in addition to the ventricular septum, the papillary muscles of the atrioventricular valves. It also persists as the apical ventricular trabeculations. During the process of development, extensive connections are present between the developing non-branching and branching components of the conduction axis and the crest of the muscular ventricular septum (Figure 6C). The inferior transition between the ventricular axis and the tricuspid ring at this stage can be seen to be marked by passage of the axis beneath the margins of the inferior atrioventricular cushion (Figure 6D). At the end of the embryonic period of development, which is 8 weeks subsequent to conception, there has been no formation of the inferior pyramidal space. Furthermore, the aortic root at this stage is supported by a muscular infundibulum, even though the interventricular communication has closed.9 It is during the ongoing stages of foetal development that the atrioventricular junctions expand to produce the inferior pyramidal space. It is also during the initial weeks of foetal development that outflow myocardium is incorporated in the left ventricular outflow tract to support the leaflets of the aortic valve. The details of these processes remain to be established. Our current knowledge does permit us to infer that the ventricular septum shares an origin from the initial trabecular myocardium with the bundle branches. Our findings also suggest that the apical trabeculations provide the transition from the insulated bundle branches to the compact myocardium of the ventricular walls (Figure 4).
Comparative Anatomy of the Conduction Axis
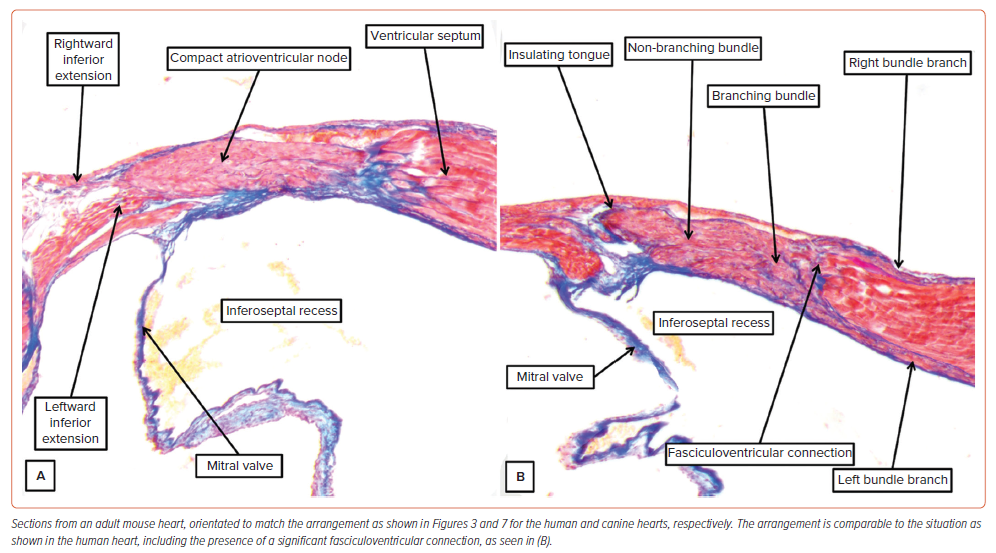
Much of the evidence regarding the behaviour of the conduction axis came from studies made in canine hearts. This is particularly the case for the experimental studies cited in favour of longitudinal dissociation in the atrioventricular axis. And the Purkinje cardiomyocytes were initially described in hearts of ungulates. Indeed, it was not until Tawara examined the ovine heart that he was able to trace the ramifications of the node and non-branching bundle into the ventricular walls.4 All of this is pertinent to the understanding of the human atrioventricular conduction axis. In the human heart, the atrioventricular node becomes the non-branching bundle as its cardiomyocytes are insulated by a tongue of fibrous tissue producing continuity between the leaflets of the mitral and tricuspid valves (Figure 3D). This arrangement is not to be found in canine, porcine, ovine or bovine hearts.10 In these species, the atrioventricular node is formed predominantly from the myocytes of the tricuspid vestibule (Figure 7B, C). The insulation from the atrial cardiomyocytes is then provided by a tongue of continuity between the tricuspid and aortic valves (Figure 7D). The non-branching bundle is much longer than found in the human heart, encircling the right side of the aortic root. Furthermore, throughout its length we found extensive lateral connections between its contained cardiomyocytes when exploring the potential for longitudinal dissociation.22 Nonetheless, we do find superior connections with the right side of the ventricular septum (Figure 7E) prior to formation of the right bundle branch (Figure 7F). Unlike the situation in the human heart, there is no formation of a membranous septum. These differences were well demonstrated by Tawara (Supplementary Material Figure 3). It is only in the hearts of ungulates that it is possible to find the swollen cardiomyocytes with limited myofibrils as initially described by Purkinje. In the human heart, the specialised cardiomyocytes are of comparable size to the cardiomyocytes of the working myocardium (Figure 4). Although the findings in the canine, porcine and bovine hearts are markedly different from the arrangement in humans, that is not the situation with the murine heart. As was emphasised by Lev and Thaemert, the atrioventricular conduction axis in the mouse is very similar to the human arrangement, although the mouse does not possess such a well-formed membranous septum.13 Nonetheless, the atrioventricular node is formed by contributions from the vestibules of the mitral and tricuspid valves, with additional septal contributions (Figure 8A). The axis is also insulated from the atrial myocardium by a tongue of fibrous tissue joining the hinges of the mitral and tricuspid valves. Having reached the crest of the muscular septum within an inferoseptal recess of the subaortic outflow tract, the axis then extends first as a non-branching component before giving rise not only to the left and right bundle branches, but also to significant superior septal pathways as described by Mahaim for the human heart (Figure 8B).27
Significance of the Anatomical Findings to Pacing the Left Bundle Branch
Those championing the role of selective pacing of the conduction axis recognise fully the need to possess an intimate knowledge of its location within the heart. Therefore, it is surprising to find these experts providing such inaccurate diagrams to show its relationship to structures such as the ventricular septum and the aortic root.2,6 As we have already discussed, these experts did, more recently, include a description of anatomy based on our dissections.7 As we have also indicated, our own knowledge has advanced since then. In part, this reflects our access to large numbers of serially sectioned histological datasets, prepared not only from human hearts but, as we have shown in this review, also from other species, such as dogs, pigs, bovines and mice. These findings show marked variations in the overall arrangement of the axis between the species, while retaining the basic structure as initially emphasised by Tawara.4 The findings in the human heart show additional marked variation between individual datasets, making it difficult to provide landmarks that are likely to be accurate in all situations. This is particularly the case for the adjacency of the axis to the aortic root, a feature also of importance to those undertaking transcatheter replacement of the aortic valve.17
Certain features regarding the location of the conduction axis remain unresolved, despite our burgeoning anatomical knowledge. As yet, it remains to be determined why the atrial components of the axis cannot be identified by catheterisation within the triangle of Koch. Is it the insulation provided to the ventricular component of the axis that permits the recording of the so-called His spike? Of greater significance, could it be that the fasciculoventricular pathways are responsible for conducting the paced impulse when seeking to correct bundle branch block?42 The superior septal pathways are ubiquitous in the murine heart, as they are in the human heart.13,26 In the murine heart, the pathways permit activation of the septum in base-to-apex fashion.43 In humans, during sinus rhythm, the pathways are dormant. As we have shown, they are able to conduct when paced at higher voltages.42 There is still much to be done before the conduction axis can be placed back into the heart with total accuracy. Identification of its exact location using techniques, such as micro-CT or synchrotron imaging, will help.44,45
Clinical Perspective
- Those concerned with pacing the conduction system have rightly pointed to the need to understand its location within the heart, but many current drawings are anatomically incorrect.
- We emphasise that, so as to understand the correct location of the conduction axis, it is preferable to use attitudinally appropriate nomenclature.
- Marked variation is to be found in the relationship between the conduction axis and the aortic root, posing challenges in providing accurate landmarks for procedures, such as transcatheter replacement of the aortic valve.
- To date, little attention has been focused on the potential significance of the superior septal pathways described by Mahaim as an alternative pathway for conduction during pacing.
- Those making inferences from experiments conducted in animal species should appreciate that the arrangement of the conduction axis differs significantly between canine, porcine and bovine hearts compared with humans, but the murine heart shows a similar arrangement.