Brugada syndrome (BrS) is a clinical entity identified in 1992 by Brugada brothers from a file of patients resuscitated from sudden cardiac death (SCD).1 Of these patients, some had a specific electrocardiogram (ECG) appearance characterised by an incomplete right bundle branch block associated with an ST segment elevation in the right precordial leads. It quickly became apparent that this disease was often familial with an autosomal dominant mode of inheritance. This ECG pattern is associated with an increased risk of sudden death resulting from polymorphic ventricular tachycardia (VT) and/or ventricular fibrillation (VF) in absence of structural abnormalities.
There is a strong male predominance (eight cases out of 10) and Brugada syndrome is usually found in patients older than forty years old.2–4 However, some cases have been described in very young children as well as in elderly people.5 BrS, initially considered as an extremely rare disease, is actually found in 4 % of patients with aborted SCD and represents 20 % of sudden deaths without underlying heart disease.3 The prevalence can be estimated around 5/10000. However, geographic disparities are observed with a higher frequency in Asia than in west Europe or North America.3,4
Diagnosis
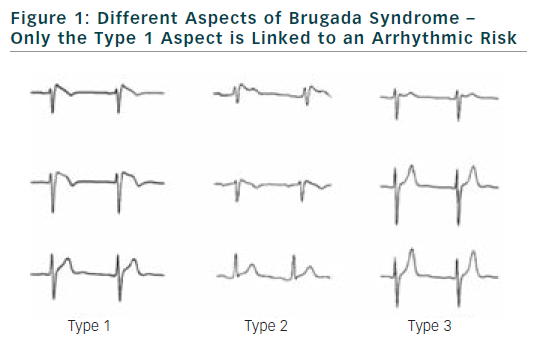
The diagnostic criteria were well specified in two consensus conferences. Diagnosis of BrS can be performed only if a ≥2 mm ST-segment elevation in two consecutive leads is observed. It is also required a convex or triangular ST-elevation followed by a negative T wave in the same leads (see Figure 1).3,4
To perform BrS diagnosis, other possible causes of ST segment elevation, like ischemia or cardiomyopathies have to be eliminated.
Diagnosis of BrS can be established either on a basal electrocardiogram or after sodium channel blockers challenge. Several drugs can be used like flecainide, pilsicainide or ajmaline. When these tests are performed according to consensus conference recommendations, risk of accidents during the test is low.6–8 The aspects of type 2 or 3 of BrS, if not converted to type 1 aspect during sodium channel blocker challenge, are not related to an increase risk of SCD.
If making the diagnosis of BrS isn’t a real problem, evaluation of the prognosis is much more difficult. Over the last years, through the establishment of large databases, it became possible to have a relatively accurate idea of the risk associated with BrS at a population level.2,9–11 For this, clinical, electrocardiographic and eventually genetic data can be used.
However, it is important to keep in mind that these criteria are only valid at the population level and they have a limited value at the individual level. Therefore, one should be extremely cautious about the risk assessment that could be given to a single patient.
Clinical Parameters
Since the identification of BrS, several clinical parameters were found to be highly predictive of arrhythmia and sudden death occurrence.
Patients with a personal history of sudden death have an annual arrhythmia’s risk as high as 10 %.2,9–12 Similarly, the presence of syncope is consistently associated with an increased arrhythmic risk.2,9–11 This risk can be estimated at about 1.5 % per year. The difficulty for the assessment of arrhythmic risk associated with syncope is based on the ability to differentiate arrhythmic syncope from simple neurally mediated syncope. This is not so easy in clinical practice because patients affected by BrS are also frequently affected by neurally mediated syncope regardless clinical presentation of BrS.12,13 However, it has been recently demonstrated that only arrhythmic syncope are linked to an increase risk of ventricular fibrillation and SCD.13 In this situation, it is important to obtain all possible information to determine the actual cause of syncope. In case of arrhythmic origin syncope, given the risk of SCD, there is no doubt that an ICD is needed. However, when doubt remains about the nature of syncope, it may be useful to perform electrophysiological studies (EPS) to search for other abnormalities that could explain syncope, or even to implant implantable loop recorders to avoid too systematically implantation of ICD in patients who do not need.14 This is especially true regarding the high rate of complications in BrS patients implanted with an ICD.15
Other parameters like sex and age are clearly related to an increase risk of arrhythmia. There is a clear risk peak for cardiac arrhythmias occurrence and sudden death at the age of 40 years old. This criterion must be taken into account when assessing the patient’s risk. Indeed, BrS is mainly diagnosed in 40-year-old men. Thus, this population is over-represented in databases leading to an over-estimation of arrhythmic risk in populations that do not correspond to this category of patients (especially women and those aged over 60 years). Follow–up information for these categories of patients remains very limited.5,16
The presence of SCD in family is usually not considered as a risk factor for sudden death albeit some controversies exist.2,9–11,17 The assessment of familial SCD role is complex because usually in cases of sudden death, family screening is carried out more completely leading to the identification of patients whose risk is lower. This bias can lead to an underestimation of overall risk in families affected by sudden death.
Electrocardiographic Parameters
Since the identification of BrS, special attention was paid to electrocardiogram analysis in an attempt to determine arrhythmic risk.
Numerous parameters have been proposed. However, it should be noted that for most of these parameters, there was no prospective evaluation and no validation in a replication population that strongly limits the use of these parameters in daily clinical practice.
Presence of a spontaneous aspect of BrS is found consistently as an important risk of arrhythmias occurrence in different databases. This is true both in symptomatic and asymptomatic patients.2,9–11 It also appears that the presence of an intermittent type 1 aspect is associated with an increased risk of arrhythmias. It is also now clear that there is no difference in term of prognosis regarding the precordial leads where ST segment elevation is found. Presence of type 1 aspect at the third or fourth intercostal space is more a matter of morphology of the patient than a modification of the electrophysiological substrate.18
Type 1 burden could be seen as a marker of interest in risk classification. It should be noted that so far, likely due to technical difficulties to evaluate over a long period this parameter, there are few data supporting use of specific tools during a Holter ECG for assessment of type 1 burden.19
Presence of an increase of ST-segment elevation during recovery from exercise testing can be a predictor of poor prognosis, especially for patients with syncope alone and for asymptomatic patients.20 Prominent R wave in lead aVR may reflect more right ventricular conduction delay and subsequently more electrical heterogeneity, which in turn could be responsible for a higher risk of arrhythmia in BrS patients.21
Presence of fragmented QRS has long been recognised as a prognostic factor of interest. The evaluation of QRS fragmentation can be performed during an electrocardiogram with signal averaged ECG.22,23 Recently, presence of fragmentation on ascending limb of QRS defined as two or more spikes within the QRS complex in leads V1 to V3 has been shown to have prognostic value.9 QRS duration seems also to have some prognosis value.24
Early repolarisation is frequently identified in patients affected by BrS. However, this aspect does not change prognosis of these patients.25 T wave alternance has also been proposed as a marker of risk. However, up to now there is little clinical information and this parameter could not be used in clinical practice.26
Repolarisation dispersion has also been involved in the occurrence of ventricular arrhythmia in BrS patients and is one of the major components of the pathophysiological hypothesis to explain BrS for both repolarisation and depolarisation hypothesis. One way to explore repolarisation dispersion is to analyse Tpeak-Tend dispersion on a surface ECG. This parameter has been related to positivity of electrophysiological study.27 Its value to determine the risk in BrS patients is more controversial.28
Role of the Electrophysiological Study
In the current literature, the predictive value of EPS is highly controversial. Indeed, while some authors report a high prognostic significance of EPS, others deny its usefulness.2,9–11,29–32 In 2005, the second consensus conference issued a Class IIa for use of EPS in patients with a spontaneous type 1 ECG and a Class IIb for EPS in patients without a spontaneous type 1 pattern.3 In 2006, the American Heart Association/ American College of Cardiology/ European Society of Cardiology guidelines for prevention of sudden death reflected the ongoing debate and did not provide stringent indication for EPS in BrS (Class IIb).33
Reasons why data is so controversial regarding the use of EPS in patients with BrS are probably multiple. These include a lack of homogeneity in protocols used for EPS, patient’s criteria selection, geographic and ethnic patient’s origin and the exhaustively of the follow-up after the EPS. Probably, the main cause of variation in EPS results remained the statistical methods used to evaluate usefulness of EPS. Indeed, the difficulty in analysing EPS value is that the results of EPS will change the management of patient. In most cases, when EPS is positive the patient is implanted with an ICD and then they will be carefully observed with frequent consultations and home monitoring. All the arrhythmic events will be recorded, even if the event is only a short run of polymorphic VT. Sometimes this can be totally asymptomatic and still be treated by the ICD with an appropriated shock.
On the contrary, in asymptomatic patients with a negative EPS, monitoring is not always performed in a tertiary center (since the information is given directly to the patient, they carry a low arrhythmic risk). In this case, complete follow-up is more difficult to obtain and probably not exhaustive in particular in patients who remained asymptomatic.
Recent results of Programmed electrical stimulation predictive value (PRELUDE) study demonstrate the prognosis value of EPS, seem to close the debate.9 Interestingly, in this study it was demonstrated that a short ventricular refractory period seems to be a strong prognosis parameter. This criterion has still to be demonstrated on an independent cohort of patients to be used in clinical practice.9
Use of Genetic to Evaluate the Risk of Arrhythmia
Twelve genes have been associated with Brugada syndrome. Involvement of SCN5A was the result of a candidate gene approach in 1998. Since, many mutations in this gene have been described in patients with BrS.
SCN5A-encoded cardiac sodium channel “loss-of-function” mutations provide pathogenic basis for an estimated 15-30 % of BrS, currently representing the most common BrS genotype.2,9,34,35 However, it must be recognised that nearly 2 % of healthy Caucasians and 5 % of non-whites also host rare mis-sense SCN5A mutations, leading to a potential conundrum in the interpretation of genetic test.35 In addition, it has recently been shown that if presence of mutations in SCN5A gene was strongly correlated with a presence of conduction disturbances, their relations with BrS were complex. Besides a low penetrance of these mutations there is a high frequency of phenocopies. These data are surprising in the context of a rare disease.36
Regarding the genotype-phenotype relationships, ECG profiles of mutated and non-mutated patients can be distinguished by the longer conduction time in patients carrying a SCN5A mutation.37
A second locus was identified in 2002 by a family approach leading finally to identification of a GPD1L mutation.38 Recently, loss-of-function mutations in cardiac calcium channel (CACNA1C) and its regulatory subunit CACNB2b were detected in patients with Brugada syndrome sometimes associated with a QT interval shorter than normal.39 It is still early to state frequency of mutations in the L–type calcium channel, although authors suggested that it would be 4.9 % in BrS patients with a normal QT duration (> 370 ms).
From two families, mutations in the SCN1B gene were identified leading to a decrease of sodium current and inducing conduction disturbances and an aspect of BrS in some patients.40 More recently, a mutation in MOG1 gene, partner of Nav 1.5, was identified.41 Several gain of function mutations have been described in genes associated with Ito current (KCNE3, KCNE5 and KCND3) in sporadic cases of Brugada syndrome.42–44 Mutations were also identified in SCN3B, KCNJ8 and CACNA2D1.39,45,46
The low frequency of the detected mutations in the genes other than SCN5A prevents the possibility to evaluate a genotype-phenotype correlation.47
Even if more data are available for SCN5A mutation carriers, up to now, there is no relation between arrhythmic risk and presence of SCN5A mutation. A recent study showed that SCN5A mutation–positive patients present a significantly longer PQ interval, and that 39 % of BrS patients with a PQ interval ≥ 200 ms carry a SCN5A mutation.47
Currently and until further studies are available, genetic analysis could not be part of arrhythmic evaluation in BrS.
Therapeutic Consideration
Medical Treatment
Currently, no medical therapy has proven its efficacy in BrS. Several case reports and retrospective studies are in favour of a possible therapeutic effect of quinidine therapy.48,49 This drug could be used in symptomatic patients implanted with an ICD in whom frequently appropriate ICD shock occurred or in children.50 Data are missing concerning use of quinidine treatment in primary prevention in non–implanted patients. Some studies showed a beneficial effect to decrease the rate of positivity of EPS.51 However, regarding the low predictive value of EPS, potential pro-arrhythmic effect of the drug and low frequency of arrhythmic event in asymptomatic patients, use of quinidine in primary prevention in asymptomatic patients could not be encouraged. Many drugs such as antiarrhythmic or some antidepressant may increase ST segment elevation and for some of them increase risk of arrhythmic events. A list of these drugs should be provided to patients.52
Arrhythmic events frequently occurred during fever episode. For this reason, patients have to be informed to actively treated fever with anti pyretic treatment.
BrS is often a family disease. It is important to investigate presence of electrocardiographic abnormalities in first-degree relatives.
ICD implantation should be regarded as the perfect treatment for BrS patients. As the only risk is occurrence of VF, ICD is the perfect device to detect and treat VF. Unfortunately, because of a high rate of complications in patients implanted with an ICD, the situation is more complex. Inappropriate shocks occurred at an annual rate of 2.5 % per year and at five years 20 % of patients experienced lead failure.15
In symptomatic patients, there is no doubt about indication for ICD implantation. Patients should be interviewed carefully for atypical symptoms such accidental nocturnal enuresis or abnormal nocturnal breathing which may be considered as an equivalent of aborted sudden death.
The question of ICD implantation is much problematic in asymptomatic patients. In asymptomatic patients without a spontaneous type 1 aspect, arrhythmic risk is low (0.3 % per year in FINGER registry).2 In this situation, risks of ICD complications clearly outweigh risks of BrS and a careful annual follow-up should be proposed to the patient.
In asymptomatic patients with a spontaneous type 1 aspect of BrS, the arrhythmic risk is around 0.8 % per year. Therapeutic decision is much more complex here. All different parameters described before should be used in order to better determinate specific patient’s risk. However, as we do not have tools that can clearly distinguish patients who will experience sudden death and those who will remain asymptomatic all their life, special attention should be paid to patient’s wishes. This is particularly important for ICD implantation and it must be explain to the patient that on a statistical point of view, it is more likely that he will experience ICD complications rather than appropriate shock. Under these conditions, it is essential to obtain good patient adherence to treatment choice, to ensure a satisfactory quality of life.53
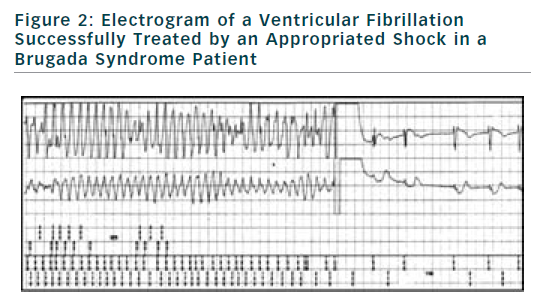
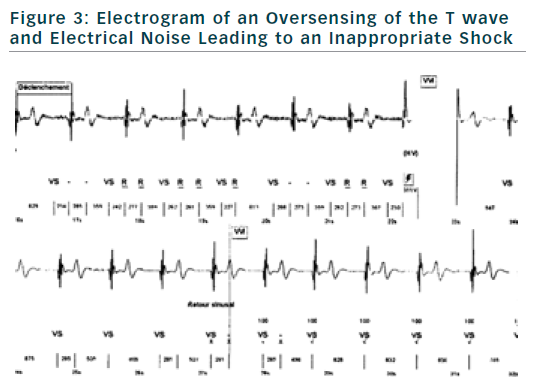
If finally an ICD is implanted, the choice of type of ICD will be preferentially VVI ICD with a monocoil active lead to facilitate future ICD lead extraction. To limit the risk of inappropriate shock, ICD program must be as simple as possible with a unique VF zone higher that 220 bpm (see Figure 2). During implantation special attention should be paid to obtain a good detection of R wave to reduce risk of T waves oversensing (see Figure 3).
In patients who frequently received electric shock, treatment with quinidine may be proposed. In case of recurrence of VF, it has been recently propose to perform ablation of the RVOT epicardium.54
Conclusion
Arrhythmic risk stratification and therefore treatment decisions remained complex in Brugada syndrome patients. In patients with an intermediate risk, it is essential to give clear information to patients, explaining that it remains many questions about the optimal management of this disease. In this situation, a careful evaluation of the arrhythmic risk using all the different tools available is mandatory.







