Ventricular tachycardia (VT) and VF occur mainly in people with impaired cardiac function and/or ischaemic heart disease, and account for the majority of sudden cardiac deaths worldwide.1 Treatment with anti-arrhythmic drugs such as amiodarone may be at best neutral in terms of mortality and carries significant long-term risks.2,3 While ICDs significantly improve survival for patients with significantly impaired left ventricular ejection fraction (LVEF), the devices also carry risks of infection and inappropriate shocks being given.4,5
Some patients may present with a ‘secondary prevention’ ICD indication such as sustained VT or VF arrest, but, in the primary prevention setting, the risk of arrhythmia is based on the presence and severity of structural heart disease. Selection of patients in this way lacks precision and fails to identify some at-risk patients while leading to overtreatment in others. Current guidelines recommend echocardiography as the first-line investigation for cardiac function due to ease of access, because the echocardiographic equipment is usually available in heart clinics, whereas cardiac MRI (CMRI) services currently tend to only be available in specialist (tertiary) centres.6 However, CMRI is superior in terms of both accuracy and reproducibility when quantifying LVEF and myocardial mass, and can overcome limitations of inadequate echocardiographic windows. CMRI offers a one-stop investigation for accurately establishing cardiac structure, function and myocardial tissue characterisation.
Understanding the Substrate for Ventricular Arrhythmia
Ischaemic Cardiomyopathy
Studies of cardiac tissue obtained before transplantation or following left ventricular (LV) aneurysm surgery, as well as more recent human and animal CMRI studies, have confirmed our understanding of the structural changes that occur in ischaemic cardiomyopathy (ICM). Strands of surviving tissue within and at the periphery of the infarct region form tortuous and slowly-conducting channels which support re-entry, so the infarct border zone frequently has a heterogeneous appearance on CMRI.8–10
Fenoglio et al. demonstrated there were bundles of surviving myocytes in endocardial resection samples; some of these had a diameter of <100 µm, but it was not known which of these channels were mechanistically important.11 De Bakker et al. showed that differential slow conduction occurs with multiple tracts <200 µm.9 Recently, ultra-high (submillimetre) resolution ex vivo CMRI of infarcted porcine hearts showed conducting pathways were mainly subendocardial.12 However, in this study, a significant minority of pathways were observed to be entirely epicardial and would be inaccessible for endocardial catheter ablation.
Urgent reperfusion (by either thrombolysis or angioplasty) for MI reduces infarct size and the incidence of subsequent chronic VT. In observational studies, VT cycle lengths were shorter, possibly suggesting a smaller circuit, in patients who had received revascularisation than in those who had not been revascularised.13–15 This would suggest that reperfusion strategies can introduce greater substrate heterogeneity within the infarcted area.
Techniques that characterise and quantify the scar border zone or identify channels could improve risk stratification and treatment planning. However, while larger conducting channels may be identified using CMRI, it is likely that others are missed because of the limited spatial resolution of current clinical imaging. Where channels are too small to be visualised, measures of tissue heterogeneity may act as surrogates for the presence of ‘sub-resolution’ channels.
Non-ischaemic Cardiomyopathy
The aetiology of VT in patients with non-ischaemic cardiomyopathy (NICM) is less well understood, partly because of the heterogeneity of underlying pathological processes in NICM. When regional fibrosis is detectable, it is often midwall or subepicardial, making access for catheter ablation challenging. These factors may explain why outcomes from NICM VT ablation are worse than those in ischaemic cardiomyopathy.16
In contrast to macro re-entrant VT, polymorphic VT or VF may occur due to distinct (but related) mechanisms. Replacement fibrosis can be patchy and/or diffuse, with disruption of the left ventricular microarchitecture.17 This diffuse fibrosis provides the substrate for conduction block and micro re-entry resulting in VF.18,19 This substrate is often dynamic with progressive fibrosis, reducing the long-term efficacy of targeted substrate modification.
Cardiac MRI Tissue Characterisation
Late Gadolinium Enhancement
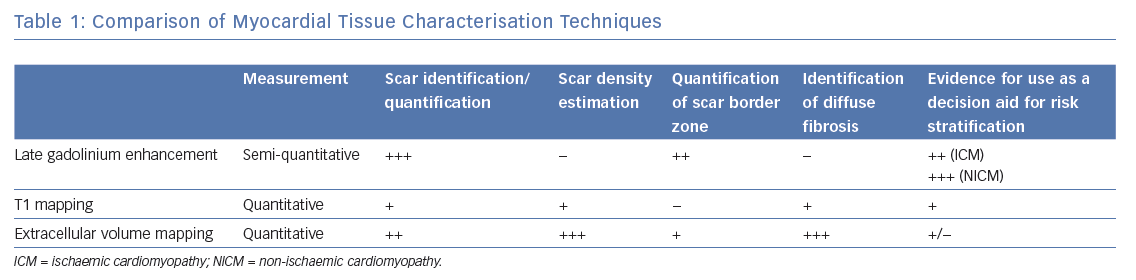
Late gadolinium enhanced (LGE) CMRI imaging has become the de facto standard for imaging myocardial fibrosis. This approach uses gadolinium as a contrast agent to highlight areas of heterogeneity within the myocardium (e.g. fibrotic versus normal areas). In normal tissue, the washout of gadolinium is rapid, whereas in areas of myocardial fibrosis the washout is slower. By timing the image acquisition to occur ‘late’ when washout has occurred in normal tissue but not in fibrotic tissue, regions of normal and fibrosed tissue can be differentiated. This technique relies on setting the inversion time to ‘null’ distant normal myocardium, making it appear black. Areas of enhancement have been demonstrated to correlate well with both acute myocardial necrosis and chronic fibrosis in ischaemic pathological specimens as well as replacement fibrosis in non-ischaemic dilated cardiomyopathy.20,21 Typical image resolution is 1.4 × 1.4 × 10 mm (the 10 mm distance is the gap between slices).
Quantification of Late Gadolinium Enhancement
Although a narrative report of scar distribution is typically given in clinical use, the volume of abnormal tissue can also be quantified based on signal intensity (SI). Manual planimetry requires the operator to manually identify areas of fibrosis, whereas semi-automated standard deviation (SD) or full width at half maximum (FWHM) techniques require less user input. The SD method defines abnormal voxels with more than 2, 3, 4, 5 or 6 standard deviations greater than the SI in a user-defined region of ‘normal’ myocardium. The FWHM method identifies tissues that fall below the SI of a user-defined area of fibrosis. Typical FWHM thresholds define a dense scar as one with >50% peak SI and a border zone between 35% and 50%.22–24 These techniques generate either a mass or percentage value of affected myocardium for the total scar burden, or for subdivisions of border zone and scar core. Although these techniques are reproducible, depending on the method and threshold chosen, significant inter-method variation is seen, and there is limited comparison with the gold standard of pathological specimens.25 In a small series, FWHM method correlates best with pathological specimens in animals and with manual segmentation in humans with ICM.24,26
T1 Mapping
Conditions with diffuse tissue fibrosis are more challenging to detect with LGE if there are no unaffected myocardial segments. Measurement of absolute T1 relaxation values sidesteps the requirement for tissue inhomogeneity in LGE imaging. Spatial resolution is inferior to LGE imaging at approximately 1.4 × 1.9 × 6 mm and is challenging at higher heart rates, though native T1 mapping does not require the use of a contrast agent.27 As imaging protocols, field strength and acquisition methods vary, reference T1 values are specific to the vendor/manufacturer.
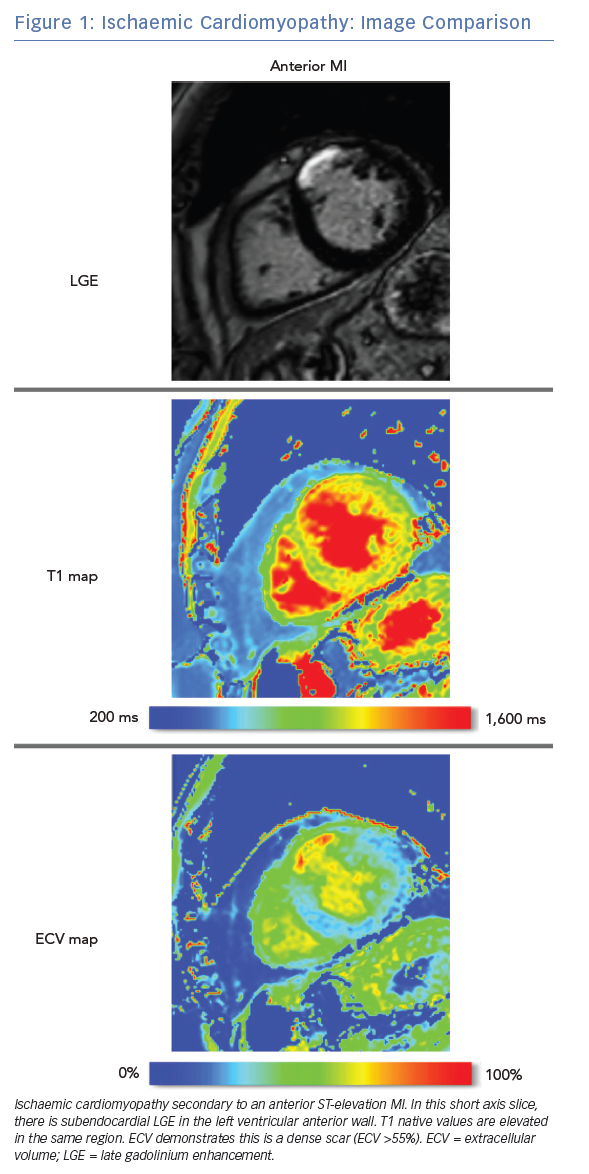
Unlike LGE, native T1 values are frequently abnormal in diffuse diseases of the myocardium, giving insights into the aetiology of NICM. T1 values are increased by tissue oedema and fibrosis, and are reduced by lipid overload (e.g. in Anderson-Fabry disease) and iron overload.28 For clinical use, mid-myocardial septal values for T1 are reported, though a map can be generated showing the native T1 values across an imaging slice. The T1 map may highlight focal areas of oedema as seen in acute myocarditis, acute myocardial infarction or takotsubo cardiomyopathy.29,30
Extracellular Volume
Contrast-enhanced T1 mapping allows the extracellular volume (ECV) to be estimated. By comparing pre- and post-contrast T1 values (referencing the T1 values of the blood pool and the patient’s haematocrit), a value for ECV is obtained. This is expressed as a fraction of the tissue volume; published normal values for ECV are approximately 25%.31,32 While native T1 values examine entire tissues, ECV characterises only the extracellular matrix and is therefore less affected by acute oedema. Higher ECV values are seen with expansion of the interstitium due to fibrosis or deposition and therefore correlate well with fibrotic changes at endomyocardial biopsy.33,34
As with native T1, ECV can be expressed as a global value or as a map highlighting regional variation. While ECV is raised in areas of chronic infarction, its main advantage over LGE for arrhythmic risk stratification is its potential to identify diffuse myocardial fibrosis in NICM.28,35
T2 Imaging
Acute myocardial injury results in interstitial oedema. This occurs rapidly after myocardial infarction, and T2-weighted CMRI sequences, which identify oedema, can predict final infarct size.36 In chronic conditions such as cardiac sarcoidosis, myocarditis, transplant rejection and toxic cardiomyopathies, T2-weighted imaging accurately identifies myocardial oedema.37 While arrhythmic complications in these conditions may be predicted using CMRI, there is limited data to support T2 imaging for arrhythmic risk stratification in patients with ICM or NICM.38
Comparison of Techniques
LGE CMRI identifies the aetiology of left ventricular systolic dysfunction (LVSD), and permits the identification and quantification of myocardial fibrosis. As a semi-quantitative technique, LGE can demonstrate only relative differences between fibrotic and non-fibrotic myocardium. As a result, diffuse diseases of the myocardium may be missed with this technique. Newer techniques such as T1 and ECV mapping have the advantage of being quantitative and, as such, can be used to identify such diffuse myocardial fibrosis seen in some forms of NICM. Table 1 demonstrates these differences. Examples of these techniques are shown in Figure 1 and Figure 2.
Current Clinical Application of Cardiac MRI
Current guidelines recommend echocardiography as the firstline investigation in patients presenting with heart failure or VT, although CMRI gains a class I recommendation if an infiltrative cause is suspected.39,40 With echocardiography or CMRI, regional wall motion abnormalities and wall thinning suggest an ischaemic aetiology, while global hypokinesis supports a non-ischaemic cause. However, assessment is highly dependent on image quality and CMRI can overcome inadequate echocardiographic windows.39 In patients presenting with VT, CMRI is particularly useful for identifying inflammatory or infiltrative aetiology as well as ischaemic and non-ischaemic cardiomyopathies. In one series, CMRI changed the working diagnosis in 50% of patients presenting with VT/VF.41 Myocardial infarction shows a subendocardial to full thickness pattern of LGE, which will conform to one or more coronary territories. Conversely, non-ischaemic dilated cardiomyopathy often has a more diffuse pattern of fibrosis.17 As a result, the location of regions highlighted by LGE in such patients is variable, but it is more commonly located in the midwall or epicardial regions of anteroseptal or inferolateral segments.42
Revascularisation of hypokinetic non-infarcted chronically ischaemic tissues may result in functional recovery.43 Hyperenhancement transmurality in LGE CMRI correlates well with myocardial recovery after revascularisation. In a series of 50 patients, regions with ≤25% transmurality were likely to demonstrate improved contractility, while those with >50% transmurality showed poor functional recovery after revascularisation.20
When myocardial ischaemia causes polymorphic VT/VF, revascularisation is indicated. However, in patients with sustained monomorphic VT, revascularisation is more contentious, since monomorphic VT usually reflects established substrate that may not be altered by revascularisation.
Indeed, in a case series of 65 patients with coronary disease and VT/VF, surgical revascularisation did not appear to affect inducibility of arrhythmia, but was associated with good long-term outcomes.44 Several other observational studies have similarly found that a reduction in mortality is associated with either PCI or surgical revascularisation in patients presenting with VT/VF.45–49
Cardiac MRI Risk Stratification
Current European Society of Cardiology guidelines for ICD implantation (in both ICM and NICM) are based upon LVEF and New York Heart Association class but not formal scar quantification.6 The more recent 2017 American Heart Association guidelines differ slightly, with a class IIa recommendation given for the use of CMRI imaging to aid risk stratification in patients with suspected NICM.40
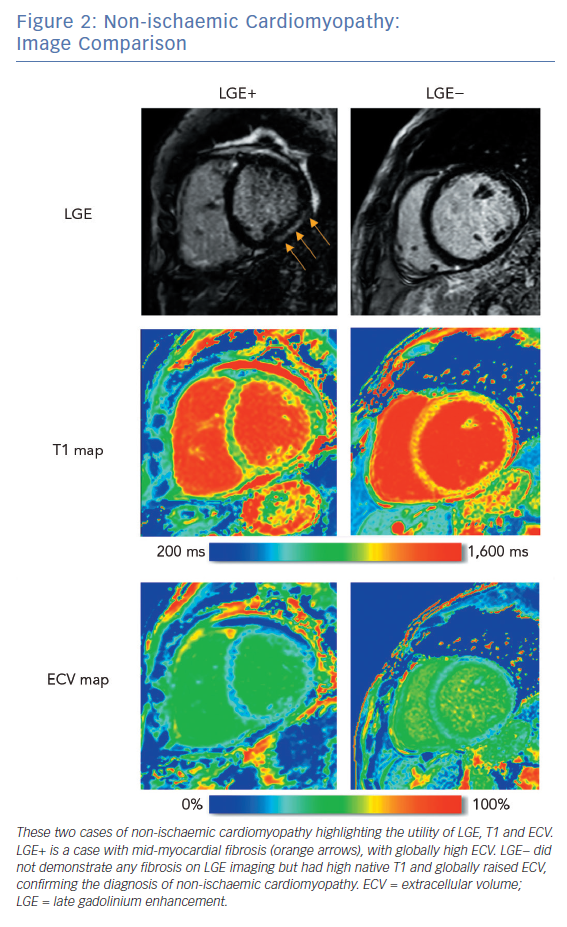
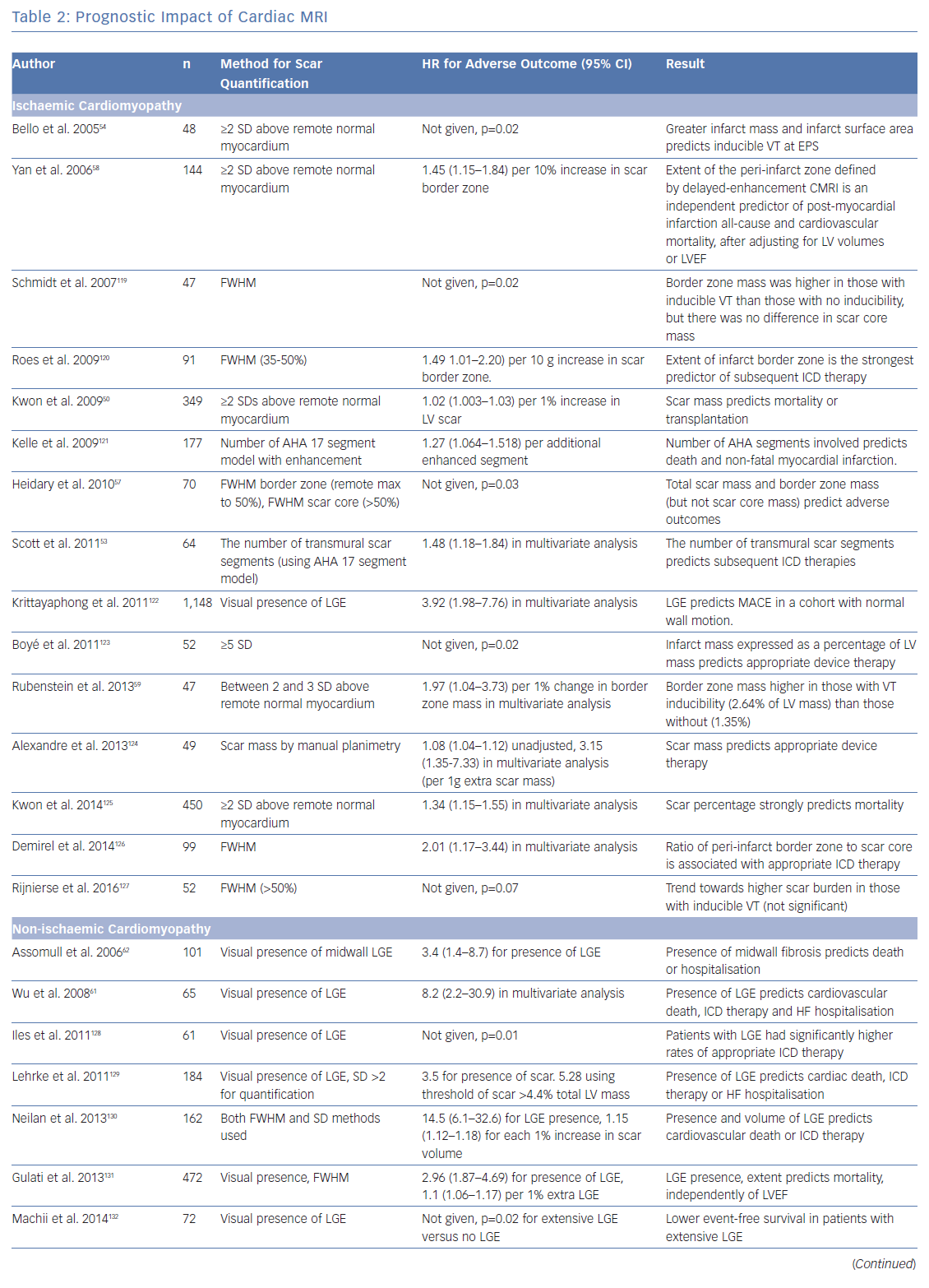
To investigate the role of CMRI as a tool for risk stratification, PubMed was searched using the terms (‘Risk Assessment’[Mesh] OR ‘Prognosis’[Mesh] OR ‘Predictive Value of Tests’[Mesh]) AND (‘Myocardial Ischemia’[Mesh] OR ‘Dilated Cardiomyopathy’[Mesh]) AND (‘Magnetic Resonance Imaging’[Mesh]) OR ‘Gadolinium’[Mesh]) to identify studies using CMRI to guide risk stratification. These studies are summarised in Table 2.
Ischaemic Cardiomyopathy
The presence of LGE with CMRI imaging strongly predicts mortality in patients with ischaemic cardiomyopathy, independently of LVEF, including in patients without detectable LVSD.50–52 Total scar burden correlates with mortality and ICD discharges, even in multivariate models including LVSD (Table 2).51–56 Quantification of the scar border zone (rather than scar core) or quantifying the number of peri-infarct channels are alternative approaches to predicting VT/VF events.57–60
Non-ischaemic Cardiomyopathy
As in ICM, the presence of LGE on CMRI in patients with NICM strongly predicts mortality and arrhythmic events across the spectrum of LV impairment.21,61–63 Patients with fibrosis identified by LGE are also less likely to achieve reverse remodelling with medical therapy.64 The spatial distribution of fibrosis is also important, with septal scarring conferring a higher risk of sudden cardiac death (SCD) than inferolateral variants, and subepicardial scarring conferring a higher risk than linear mid-wall fibrosis.65
Although patients with a low LVEF (<35%) have the highest individualised risk of SCD, this accounts for only ~20% of all cardiac arrests. The great majority of cardiac arrests occur outside this high-risk category.1 Patients with fibrosis identified by LGE have worse outcomes than those without and risk stratification of individuals based on the presence or absence of LGE rather than on LVEF alone may aid patient selection for ICDs.66 For example, compared with all those with LVEF <35% (i.e. using echocardiographic risk stratification alone), those with an LVEF >35% and LGE have similar risks of SCD.63 Moreover, these selected patients with preservation of pump function will often have a lower competing risk of non-arrhythmic death.
In contrast with ICM, where scar-related monomorphic VT predominates, patients with NICM are more likely to experience polymorphic VT and VF.67 On a review of the literature, most studies examining CMRI for risk stratification in NICM do not differentiate between VT and VF (Table 2). This practical approach is helpful for treatment decisions. However, Piers et al. found that scarring predicts monomorphic VT but not polymorphic VT or VF, suggesting that factors other than macroscopic anatomical substrate may be important in arrhythmogenesis in NICM.68
Patients with NICM with no evidence of fibrosis on CMRI have fewer arrhythmic events, a lower risk of death and a higher likelihood of reverse remodelling. Careful patient selection for prophylactic ICD implantation in this population is required, and it therefore seems logical that identification of fibrosis using CMRI could more accurately identify those who would benefit, particularly patients with NICM and those with an LVEF >35%. However, no trial data exist to supports this approach.
Late Enhancement
There is now persuasive evidence that quantification of the scar and/or border zone burden can be used to help risk stratify patients with both ICM and NICM, in addition to measures of LVEF. The fact that this relationship exists across the range of LVSD suggests that fibrosis itself is an important determinant of arrhythmic risk, rather than being simply a marker of end-stage disease.
While the presence of any degree of LGE predicts risk in both NICM and ICM, quantification of scar extent only appears to add substantial incremental risk prediction benefit in patients with ICM. However, the clinical applicability of fibrosis quantification is limited by a lack of consensus over which scar metrics and thresholds are the best predictors of outcomes, or how to apply these metrics to individuals.69
Extracellular Volume and T1 Mapping for Risk Stratification
Alternative metrics, such as native T1 values and ECV, measure diffuse myocardial fibrosis. In patients with both ICM and NICM, myocardial T1 values (at sites spatially discrete from areas of LGE) incrementally improved risk stratification in a model that already included LVEF, QRS duration, and metrics of scar core and border zone (using LGE).70 In a similar study using ECV rather than T1, high ECV values correlated with mortality.71 In two small case series, high ECV values correlated with ICD therapies.35,72 These studies suggest that, when dense scar is surrounded by diffusely fibrotic myocardium, VT/VF is more likely than if the scar is encompassed by normal myocardium.
ECV and T1 mapping techniques have a sound physiological basis for identifying diffusely abnormal myocardium not identified with LGE imaging. ECV is of particular interest as a marker of risk in patients with NICM who do not have identifiable LGE, since it offers the ability to identify diffuse interstitial fibrosis. Complementary assessment of diffuse and regional disease by ECV mapping and LGE respectively may provide incremental benefit for risk stratification in both ICM and NICM. ECV may also have value in further characterising the density of discrete scars, although data to support this use are limited.
Ablation for Ventricular Tachycardia
For patients with a high burden of VT, catheter ablation can successfully reduce ICD shocks.73–75 These procedures can be challenging, with significant morbidity and mortality, since VT is frequently poorly tolerated and precise localisation of re-entrant circuits using traditional electrophysiological techniques is often challenging. VT ablation therefore often targets the myocardial scar substrate.76 Differing approaches to substrate ablation have been described – linear transection, core isolation, scar homogenisation or abolition of late potentials.77–80 Often, this requires extensive, time-consuming ablation in haemodynamically fragile individuals, which could be streamlined with a more detailed appreciation of the underlying substrate. CMRI can be used to predict the location of re-entrant circuits and channels within the scar to guide ablation lesions, the success of which can be predicted by computer modelling.81,82
Planning
The configuration of LGE on CMRI allows the operator to estimate the likelihood of successful ablation and identify whether epicardial access is required. Predominantly subendocardial ischaemic scar-related VT is usually treatable with endocardial ablation.75 Conversely, VT ablation in NICM may be hampered by inaccessibility of the substrate, and epicardial access may be required for patients with inferolateral and/or subepicardial scarring.83 Epicardial access is typically not required for patients with VT originating from a septal intramural scar, although outcomes from ablation of ‘deep’ substrate are poorer, as might be expected.84
Image Fusion
Conventional 3D electroanatomical maps (EAMs) generated during ablation procedures may be inaccurate because of poor catheter contact or reach, and contact mapping of entire cardiac chambers is time consuming.
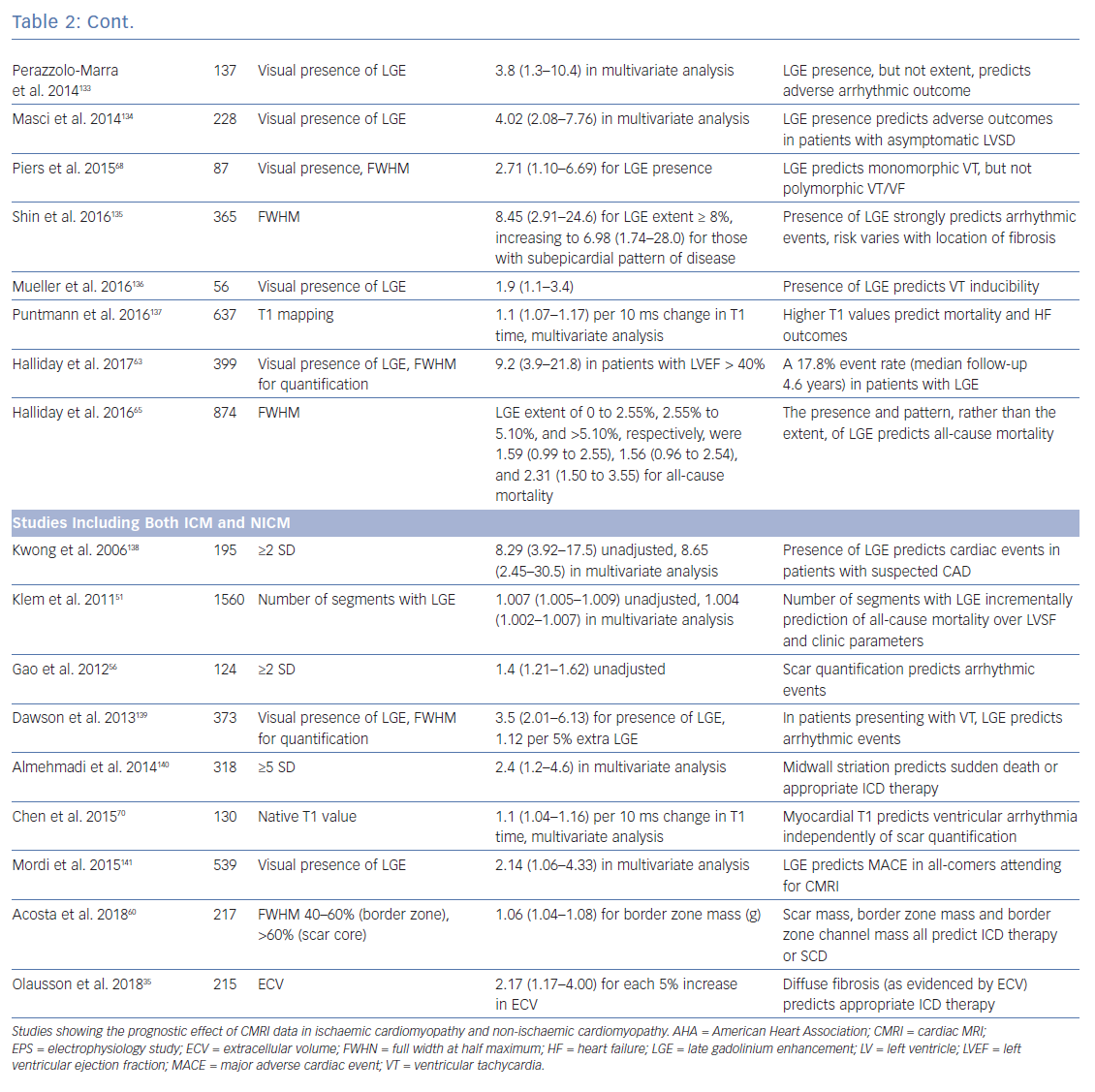
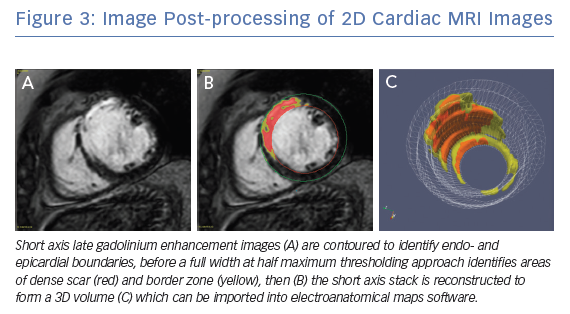
Clinical CMRI studies can be reconstructed into 3D geometries demonstrating the distribution of a scar (Figure 3). With further refinement using 3D acquisition and image-processing methods, channels that might facilitate re-entry can be identified in advance (Figure 4).85 These geometries can be used simply as a road map for the operator during ablation procedures. Alternatively, fusion of these 3D geometries with the EAM system can leverage the accurate and high resolution anatomical detail of clinical imaging, allowing the operator to observe CMRI (and/or CT) images directly in the mapping software to reduce the time spent generating EAMs.86–89 Contact mapping can be focused on regions of interest determined in advance, e.g. by using algorithms for localising the VT origin based on 12-lead ECG morphology or by non-invasive mapping (ECGI) techniques.90–95 While image fusion has the potential to streamline ablation procedures, as yet, the benefits of such an approach have not been formally evaluated, and widespread applicability is not assured since it requires significant clinical and imaging expertise.
Future Directions
Overcoming Technical Limitations of Cardiac MRI
Many patients at risk of VT/VF have cardiac implantable electronic devices (CIEDs).96 Historically, MRI has been contraindicated in patients with CIEDs due to safety concerns. However, with advances in CIED technology such as MRI-conditional devices, growing experience and appropriate precautions and monitoring, CMRI can often be performed safely even in patients with historic non-conditional devices.69,97,98 Nevertheless, images may be significantly degraded by the presence of CIEDs, particularly the anteroseptal regions of the left ventricle in patients with left-sided pulse generators that lie in close proximity to the heart. Wideband sequences are described which can reduce these artefacts.99
LGE imaging is usually obtained by multiple short axis planes through the heart. This results in excellent in-plane resolution, but a large slice width (approximately 10 mm) between images. Reconstructions of the heart can suffer with a ‘partial voluming’ artefact that can overestimate the infarct border zone.100 This effect can be mitigated by evolving techniques such as 3D image acquisition or super-resolution image reconstruction.101,102
Histological studies have demonstrated myocyte fibre disarray at the border zone of a chronic infarction.103 Due to anisotropic conduction of myocytes, knowledge of fibre orientation is potentially important to understand propensity to arrhythmia. Diffusion tensor imaging can demonstrate fibre direction and may therefore inform computer models of arrhythmia, although this use of CMRI is in its infancy.12,104,105
Ventricular Tachycardia Stimulation and Modelling
Inducibility of VT during an electrophysiology study (EPS) by programmed ventricular stimulation (PVS) pacing from a right ventricular site predicts arrhythmic events in ICM.106 This meta-analysis demonstrated PVS had the power to predict subsequent arrhythmic events (pooled OR 4.00, 95% CI [2.30–6.96]). Depending on patient selection and the number of extrastimuli used, the sensitivity, specificity and predictive value of this test varies, although is not commonly used clinically due to its invasiveness, cost and insufficient negative predictive value. In NICM, assessment with PVS is less well studied and probably less effective than with ICM.107
Scar-related re-entry often relies upon functional block as well as anatomical barriers to conduction.108 Scar quantification methods do not account for these complex mechanisms, but computer modelling has the potential to improve risk stratification by combining a personalised anatomical model with simulation of tissue electrophysiology. This method allows simulated PVS performed from multiple sites in both ventricles. In a retrospective study of 41 patients with severe LVSD, by comparing these patient-specific simulations with clinical outcomes, a positive ‘virtual-heart arrhythmia risk predictor’ simulation was associated with adverse outcomes (OR 4.05 (95% CI [1.20–13.8]), which is similar to published results from invasive PVS. Work is ongoing to determine the utility of such simulations in preserved LVSF.82,109
Simulated PVS methods are computationally significantly more challenging in NICM where myocardial fibrosis is less confluent and more heterogeneous, and the microscopic nature of the substrate is difficult to fully characterise with clinical imaging. Moreover, the substrate in NICM is often progressive and, as such, risk stratification at a single time point may fail to accurately estimate lifetime risk.
These methods are promising but are potentially limited by simplifications and assumptions in models of cell and tissue electrophysiology, the computational resources required, and the resolution of currently available clinical imaging. Despite encouraging preliminary studies, there are significant obstacles to be overcome before these approaches can be used routinely in clinical practice.110 Constructing a personalised computational model of anatomy and electrophysiology requires calibration from clinical images and data that are often noisy and incomplete, so methods for embedding uncertainties and variability into computational models are an area of active research.111 Whether these approaches can be used to guide ICD implantation in the future remains to be seen. Technological advances in imaging and modelling, along with clinical studies of their utility, will help advance this promising concept.
Future Clinical Studies
Tissue characterisation to determine who needs and, perhaps more importantly, who does not need an ICD is a complex but evolving field. Estimates of risk currently do not allow for disease progression, and it is unclear how frequently investigations should be repeated, particularly for the dynamic substrate that occurs in some forms of NICM. The effect of dynamic conditions such as electrolyte disturbance, volume overload and myocardial ischaemia on arrhythmic risk remains unknown.
In the DANish Randomized, Controlled, Multicenter Study to Assess the Efficacy of Implantable Cardioverter Defibrillator in Patients With Non-ischemic Systolic Heart Failure on Mortality (DANISH) trial (NCT00542945), investigators found no overall mortality benefit for primary prevention ICD implantation in patients with NICM.112 However, outcomes were improved by ICD implantation for those in prespecified subgroups – namely younger patients and those with lower levels of N-terminal pro-brain natriuretic peptide (NT-proBNP) – who, presumably, had a lower risk of non-sudden death. Since CMRI studies have consistently demonstrated a higher arrhythmic burden in those with evidence of LGE, a clinical trial that used CMRI-based risk stratification in NICM patients with LVEF <35% would provide clinically useful information.
Similarly, the Cardiac Magnetic Resonance GUIDEd Management of Mild-moderate Left Ventricular Systolic Dysfunction (CMR_GUIDE) trial (NCT01918215) will identify patients who have evidence of LGE but do not qualify for ICD treatment under current guidelines (LVEF 35–50%), to determine whether prophylactic ICD implantation is beneficial.113 The Programmed Ventricular Stimulation to Risk Stratify for Early Cardioverter-Defibrillator (ICD) Implantation to Prevent Tachyarrhythmias Following Acute Myocardial Infarction (PROTECT-ICD) trial will examine whether a multiparametric risk stratification algorithm (including echocardiography, CMRI and PVS) used post-infarction will identify those who may benefit from early ICD implantation.114
Contribution to Novel Therapies
Recent developments in CMRI and electrophysiology mapping systems have shown real-time tracking and visualisation of catheter position during ablation procedures to be feasible and safe for an ‘anatomical’ ablation of the cavo-tricuspid isthmus.115,116 Advantages of such a system include 3D visualisation of catheter position within complex anatomical structures (including the ability to see surrounding structures) and real-time lesion evaluation. This technology has the potential to improve outcomes in ablation procedures, but significant technological challenges remain for its use in ventricular arrhythmia.
Stereotactic body radiotherapy has recently been reported as a novel, non-invasive treatment for VT.117,118 It is dependent on accurate anatomical localisation of arrhythmic substrate to determine the radiotherapy target. CMRI imaging is the ideal modality for treatment planning.
Conclusion
CMRI imaging can accurately quantify cardiac function, and characterise the myocardial substrate to refine risk stratification to identify people who may benefit from ICD implantation and revascularisation. Although large-scale trials in this area are required, it is likely that measures of scar quantification will become increasingly recognised by guidelines in future.
A multiparametric approach using imaging and other criteria may provide the most accurate risk assessment in the future, although the interaction between each of the metrics discussed is complex and requires careful study. Advanced techniques such as automated image segmentation and channel detection, or computer simulation of electrophysiology, offer significant potential, but are still in the early stages of development.
Significant challenges remain in overcoming technological barriers and understanding how best to use the considerable information gained from a CMRI study. Nevertheless, CMRI offers clinicians and researchers an increasingly comprehensive way to diagnose, risk stratify and tailor the treatment of patients with cardiomyopathy.
Clinical Perspective
- Cardiac MRI (CMRI) is the gold standard imaging modality for ejection fraction and myocardial tissue characterisation.
- CMRI evidence of fibrosis independently predicts arrhythmic risk, even in multiparametric models which include clinical risk factors and ejection fraction, in both ischaemic and non-ischaemic cardiomyopathies.
- CMRI can be used to inform and guide ablation procedures by characterising the ventricular tachycardia substrate.
- Novel metrics such as extracellular volume mapping and channel identification have the potential to aid the electrophysiologist and provide a more robust method of risk stratification.