This session explored the underlying cause of atrial abnormalities by looking downstream of the atria at the ventricles. The recognition of focal triggers provided the earliest and strongest clinical insights into electrophysiology and the electropathology of AF, and the session reviewed the current understanding of mechanisms of focal triggers. The session ended with a reconciliatory overview of current AF mapping data from different techniques, from inside and outside – and even through – the walls of the heart.
Role of the Ventricle in Atrial Fibrillation
Dr Rohan Wijesurendra and colleagues at Oxford University have designed a prospective, longitudinal clinical study of patients with AF undergoing catheter ablation to help answer the question, “What is the precise nature of the link between AF and left ventricular dysfunction?”3 The study excluded those with inadequate ventricular rate control in AF and significant cardiovascular comorbidities to focus on ‘lone’ AF. The study also included several matched control subjects in sinus rhythm (SR). The researchers used cardiac magnetic resonance as a gold-standard method for determining left ventricular volumes and systolic function. The results showed:
- In patients with AF, left ventricular (LV) function and myocardial energetics are impaired compared to matched controls in SR.
- Before ablation, AF at the time of the scan (compared to SR) is associated with lower left ventricular ejection fraction (LVEF), but energetics are equally impaired irrespective of the intra-scan rhythm.
- Post-ablation, LVEF improves modestly, but, like left atrial ejection fraction, does not normalise.
- The improvement in LV function is driven by the switch to SR at the time of the scan, rather than by a reduction in AF burden over time.
- Myocardial energetics are unchanged post-ablation, despite the substantial and sustained reduction in AF burden.
The study is the first to show that lone AF is associated with impaired LV function and energetics that fail to normalise after successful ablation.
These findings suggest that apparently lone AF may actually be the consequence, rather than the cause, of an underlying cardiomyopathy. This has potential implications for AF management – just as, after plaque stenting, statins and lifestyle modification are used to target the underlying pathobiology of coronary artery disease, it may be that adjunctive therapies beyond catheter ablation are needed in AF to target the ongoing drivers of the disease process.
Focal Triggers
Dr Nattel considered focal triggers as an aspect of mechanisms of AF, and provided some new perspectives on this topic. He began with a simplified scheme of arrhythmia mechanisms in AF, involving ectopic activity, triggers and a re-entry substrate. Over 90 % of AF episodes are initiated by premature atrial contractions, which act as initiators on a vulnerable substrate.4 However, in addition, sustained ectopic activity can itself lead to AF.
Both long-standing persistent AF and paroxysmal AF patients manifest delayed afterdepolarisations due to abnormal calcium handling, but with distinct mechanisms. Novel mechanistic approaches may need to be developed to target patient-specific pathophysiology. Importantly, delayed afterdepolarisations and triggered activity are likely the principal source of atrial premature complex generation.
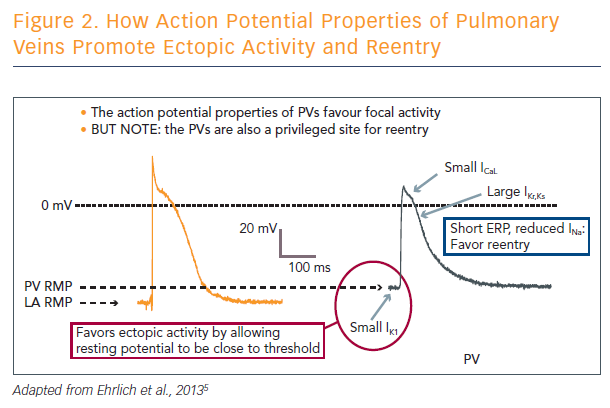
Triggered activity due to abnormal calcium handling is the principal contributor to atrial focal ectopic firing. The pulmonary veins are a privileged site for ectopic activity, but are also predisposed to supporting re-entry. In addition to their structural properties, the pulmonary veins have ion channel properties that favour ectopic firing. It is important to note that the structural and ion channel properties of the pulmonary veins also predispose them to re-entry (see Figure 2).
Not all focal firing is due to focal ectopic activity, as shown by the use of wave mapping. A recently published paper delineates the zones of epicardial and endocardial dissociation along the opposite surface, resulting in breakthrough activity.6 Thus, focal firing on the endo- or epicardial surface may be due to breakthrough instead of ectopy, and endo-epicardial interaction may multiply AF-maintaining sources. This may be a form of AF-maintaining activity that does not involve true ectopy or re-entry.