Burden of Atrial Fibrillation
Atrial fibrillation (AF) is the most common sustained arrhythmia in clinical practice, accounting for approximately one-third of hospitalisations for cardiac rhythm disturbances.1 Between 1980 and 2000, the age-adjusted incidence of AF significantly increased from 3.04 to 3.68 per 1,000 person-years in the US.2 The prevalence of AF was lower among African Americans than among Caucasians,3 and it also seemed to be lower in the Asian population.4,5 In a nationwide cohort of 702,502 participants in Taiwan, the AF incidence was around 1.5 per 1,000 person-years.6 Since a considerable number of AF patients was paroxysmal in nature, the incidence and prevalence of AF could be significantly underestimated.
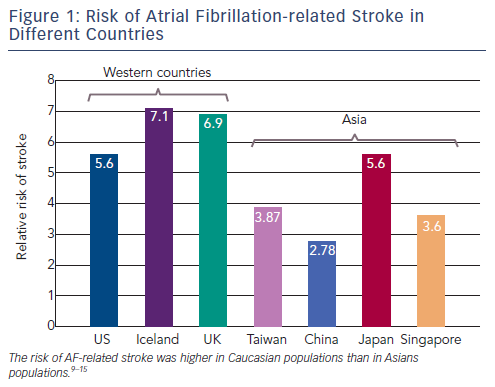
Systemic thromboembolism is the most severe complication of AF. It accounts for about 15-20 % of ischaemic strokes.7 AF-related strokes were associated with a poor prognosis as more than 50 % of the survivors remain with a severe deficit, and recurrence may be as high as 12 % per year.8 The risk of AF-related stroke was higher in Caucasian populations than in Asians populations (see Figure 1).4,9-15 AF could in fact drive a prothrombotic or hypercoagulable state, by virtue of its fulfilment of Virchow's triad for thrombogenesis (blood stasis, endocardial dysfunction/damage, and abnormal haemostasis).16 Therefore, stroke prevention with oral anticoagulants (OACs) is central to the management of AF.
The Usefulness of the CHA2DS2-VASc Score in Identifying 'Truly Low-risk' Patients
The most important point in determining the strategy of stroke prevention for AF is how to estimate the thromboembolic (TE) risk accurately. The CHADS2 score is the most commonly used scheme in stroke risk stratifications for AF patients,17 despite the fact that it classifies a large proportion of patients as being at 'intermediate risk', and several important TE risk factors were omitted in the scoring system.18 Recently, a newly developed scoring system, CHA2DS2-VASc score, which extends the CHADS2 scheme by considering additional stroke risk factors (vascular diseases and female gender) was recommended to be used to guide the antithrombotic therapies for AF patients.19,20 The CHA2DS2-VASc score is most useful in identifying truly low-risk patients, and no antithrombotic therapy is necessary for patients with a CHA2DS2-VASc score of 0.21-23
In the study performed by Taillandier et al., which enrolled a total of 616 AF patients with a CHA2DS2-VASc score of 0, an OAC was prescribed on an individual basis in 273 patients (44 %), antiplatelet therapy alone in 145 patients (24 %), and no antithrombotic therapy in 198 patients (32 %).22 During a follow-up of 876 ± 1,135 days, 38 patients experienced adverse events (10 stroke/thromboembolism, 19 major bleeding, 17 deaths). Prescription of OACs and/or antiplatelet therapy was not associated with an improved prognosis for stroke/thromboembolism (relative risk: 0.99, 95 % confidence interval: 0.25-3.99, p-value: 0.99), nor improved survival or net clinical benefit (combination of stroke/ thromboembolism, bleeding and death). More recently, a nationwide cohort study in Taiwan further demonstrated that AF males with a CHA2DS2-VASc score of 0 have a truly low-risk for stroke, which was similar to that of non-AF patients (1.6 versus 1.6 %, p-value: 0.92) during a follow-up of 57.4 ± 35.7 months.24 In the same study, the annual stroke rate was around 0.92 % for AF females with a CHA2DS2-VASc score of 1 (only due to gender),24 which was lower than that of life-threatening bleeding of dabigatran use in the Randomized Evaluation of Long Term Anticoagulant Therapy (RE-LY) study (1.22 % per year for dabigatran 110 milligrams [mg]; 1.45 % per year for dabigatran 150 mg).25 It should be emphasised that the bleeding risk in RE-LY is under ideal, clinical trial circumstances, and the bleeding rate could probably be even higher in real life. Therefore, OACs may not be necessary for AF females who are younger than 65 years of age and have no significant co-morbidities when weighing the risk and benefit of oral anticoagulant therapies. These evidences further support the recommendation of the 2012 focused updated of the European Society of Cardiology (ESC) guideline suggesting that anticoagulation is not necessary for male patients with a CHA2DS2-VASc score of 0 and female patients with gender alone as a single risk factor (still a CHA2DS2-VASc score of 1) if they fulfil the criteria of 'age <65 and lone AF'.26
How to estimate the risk of bleeding is also an important issue when determining the strategy for stroke prevention in AF patients. The Hypertension, Abnormal Renal/Liver Function, Stroke, Bleeding History or Predisposition, Labile International Normalized Ratio, Elderly, Drugs/Alcohol (HAS-BLED) score was derived from the AF population enrolled in the Euro Heart Survey,27 and was recommended to be used to evaluate the bleeding risk of OAC therapy in the ESC guideline.26
Long-term Thromboembolic Events After Catheter Ablation of Atrial Fibrillation
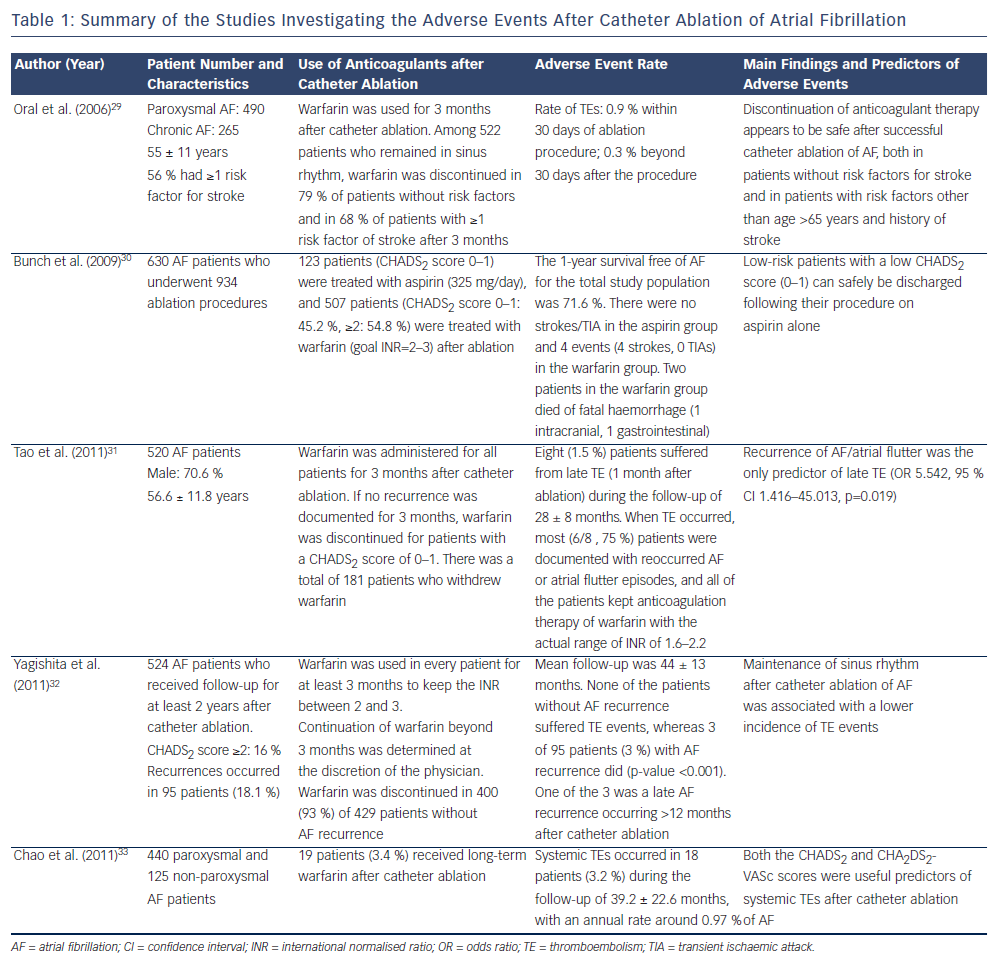
Since the popularity of catheter ablation of AF continues to escalate,28 understanding the incidence and predictors of TE events after AF ablation is important in determining the strategy of stroke prevention after ablation procedures. Several studies have reported the rate of TE events after AF ablation, which are summarised in Table 1.29-33 Although these studies differed in patients' CHADS2 scores, strategy of the use of anti-thrombotic agents and ablation procedures, the annual rate of systemic thromboembolisms was lower than 1 % in these investigations. Recurrence of atrial arrhythmias after catheter ablation was an important predictor of TE events, and maintenance of sinus rhythm was beneficial. It may emphasise the advantage of catheter ablation, which can achieve sinus rhythm more effectively for AF patients compared with antiarrhythmic drugs. In the study performed by Chao et al., which enrolled a total of 565 patients receiving catheter ablation,33 CHA2DS2-VASc score was proved to be a useful scheme in predicting adverse events independently from AF type, ablation outcome, left atrial size and left ventricular ejection fraction, and was helpful in identifying patients at risk of adverse events among those with a CHADS2 score of 0 or 1. The result of this study further validated the usefulness of the CHA2DS2-VASc score in predicting TE events in AF patients after catheter ablation.
Could Catheter Ablation of Atrial Fibrillation Reduce Long-term Cardiovascular Events?
Catheter ablation is clearly superior to antiarrhythmic drugs regarding sinus rhythm maintenance,34 and may improve quality of life of patients. In the study performed by Fichtner et al., which enrolled a total of 133 AF patients, they demonstrated that the quality of life improved significantly three months after ablation in all patients (regardless of ablation success or AF type) and stayed significantly improved after several years.35 However, the effects of catheter ablation on long-term cardiovascular events and mortality were less understood. Bunch et al. compared the risk of adverse events among 4,212 consecutive patients who underwent AF ablation, 16,848 age/gender matched controls with AF and 16,848 age/gender matched controls without AF.36 The results showed that AF ablation patients have a significantly lower risk of death, stroke and dementia in comparison with AF patients without ablation, which suggested AF ablation may eliminate the increased risk of death and stroke associated with AF. In another study performed by Lin et al., which investigated the effects of catheter ablation on long-term major adverse cardiovascular events (such as stroke/transient ischaemic attack, acute coronary events, peripheral embolism and death),37 a total of 174 patients undergoing AF ablation (minimal CHA2DS2-VASc score of 1) were matched with 174 patients receiving medical treatment using the propensity scores. They demonstrated that in AF patients with a CHA2DS2-VASc score of ≥1, catheter ablation of AF reduced the risk of the total/cardiovascular mortality and total vascular events. However, the above studies were retrospective in nature with insoluble limitations, and further prospective trials, such as Catheter Ablation versus Antiarrhythmic Drug Therapy for Atrial Fibrillation (CABANA) and Early treatment of Atrial fibrillation for Stroke prevention Trial (EAST), are necessary to confirm these findings.
Other Predictors of Cardiovascular Events, Which Are Not Included in the CHA2DS2-VASc Scoring System
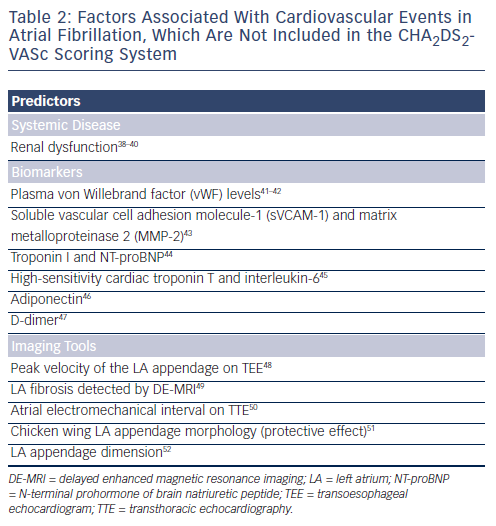
In addition to the clinical parameters included in the CHA2DS2-VASc scoring system, several predictors of adverse events were identified and may potentially refine clinical risk stratification in AF (see Table 2).38-52 Renal dysfunction was demonstrated to be an important risk factor of strokes in AF patients, although it was not included in the CHA2DS2-VASc scheme.38,39 Since patients with renal dysfunction have a high risk of major bleeding despite good anticoagulation control,53 renal function may not be helpful in identifying patients who should receive anticoagulation therapy. On the contrary, normal renal function may be useful in selecting patients with truly low-risk of TE events, and anticoagulation therapy may not be necessary for these patients. In a recent study, Chao et al. investigated the association between renal dysfunction, defined as an estimated glomerular filtration rate <60 millilitres per minute (ml/min) per 1.73 square metres (m2), and the risk of systemic thromboembolisms in 547 patients receiving AF ablation.40 They found that among patients with a CHA2DS2-VASc score of 0 or 1 and with no renal dysfunction, the TE event rate was only 0.3 %. The result suggested that it may be safe to discontinue OACs for these patients after catheter ablation of AF. Therefore, whether the combination of renal function and CHA2DS2-VASc scheme could improve the ability of the CHA2DS2-VASc score alone in selecting truly low-risk patients deserves further investigation. Several biomarkers and parameters derived from different imaging tools were reported to be associated with adverse events in AF patients. In the RE-LY sub-study, elevations of troponin I and N-terminal prohormone of brain natriuretic peptide (NT-proBNP) are common in patients with AF and independently related to increased risks of stroke and mortality.44 Similarly, a recent study from Spain showed that high-sensitivity cardiac troponin T and interleukin-6 could provide prognostic information that was complementary to clinical risk scores for prediction of long-term cardiovascular events and death.45 However, how these biomarkers could change the current strategy of stroke prevention in AF remains unknown.
Several recent studies focused on the potential role of parameters derived from imaging tools, such as echocardiography and delayed enhanced magnetic resonance imaging, in predicting TE events in AF. Nevertheless, most of these studies were single-centre observations and further validations are necessary.
Conclusion
In summary, risk stratification and adequate thromboembolism prophylaxis is the cornerstone of treatment in patients with AF. The CHA2DS2-VASc score is powerful in selecting truly low-risk patients who do not necessarily need to receive anticoagulation therapies. It is also useful in predicting TE events and mortality for patients undergoing AF ablation. Recently, more and more biomarkers and imaging parameters were reported to be associated with adverse events in AF patients. How could these biomarkers and imaging tools change the current strategy of stroke prevention in AF remains unknown and deserves further investigations.