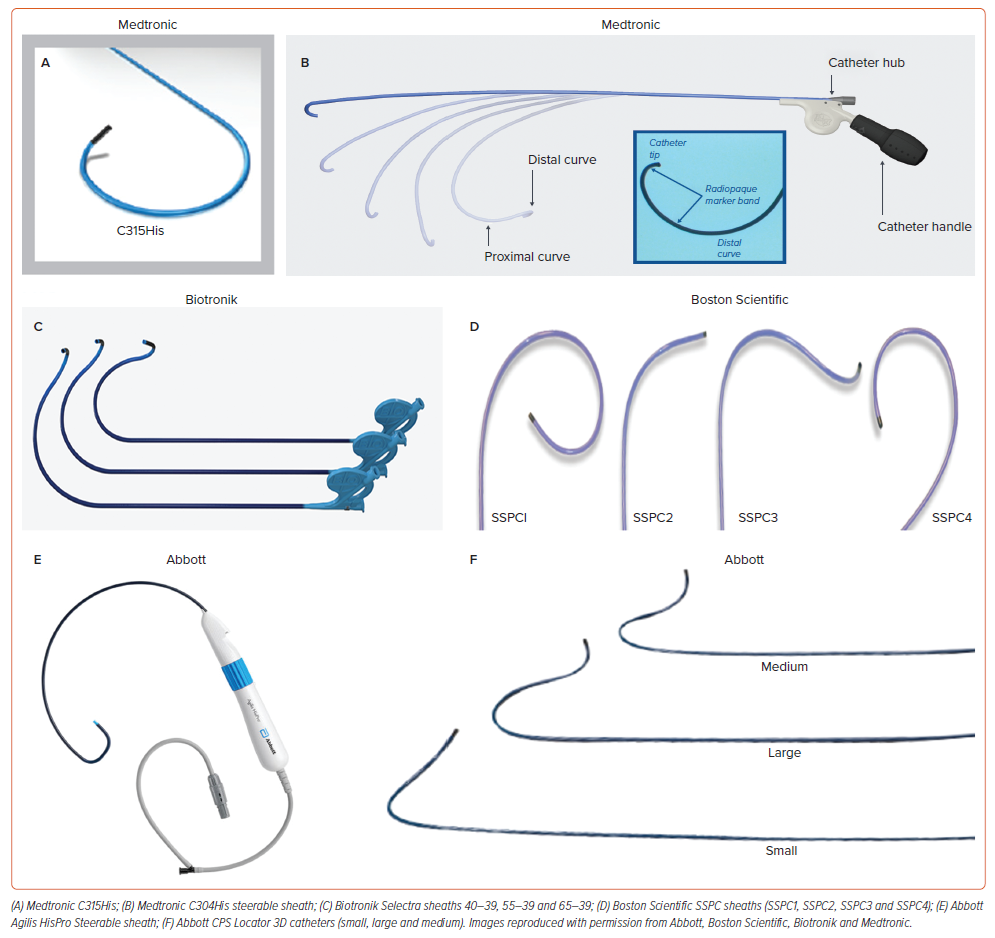
Stylet-driven leads (SDLs) have been recently used for conduction system pacing (CSP). Most of the initial experience for both His bundle pacing (HBP) and left bundle branch area pacing (LBBAP) has been obtained using the Medtronic SelectSecure 3830 lead, which is a 4.1 Fr lumenless lead (LLL) with a 1.8 mm fixed electrically active helix originally designed for selective site pacing and delivered through the C315His sheath.1–10 During recent years, commercially available conventional stylet-driven leads, with extendable-retractable electrically active helixes, have been successfully used for CSP through specifically designed delivery sheaths from various manufacturers (Figure 1).11–14 SDLs are mainly used for LBBAP, whereas only a minority of physicians use them for HBP on a regular basis. While the 3830 lumenless lead has both Conformité Européenne (CE) and Food and Drug Administration (FDA) approval for LBBAP, among SDLs only the Biotronik Solia S lead and the UltiPace lead from Abbott currently have CE and FDA approval for LBBAP, respectively. The distinctive SDL design and structural characteristics in comparison with the 3830 lead makes implant procedure workflow and lead performance different and may even determine a dedicated learning curve. The acute and long-term response to mechanical stress of LBBAP leads positioned deep inside the interventricular septum may also be different depending on lead design and structure. This review is focused on the description of the implant tools and techniques as well as the comparison of the clinical success, outcomes and complications between SDLs and LLLs.
The Learning Curve
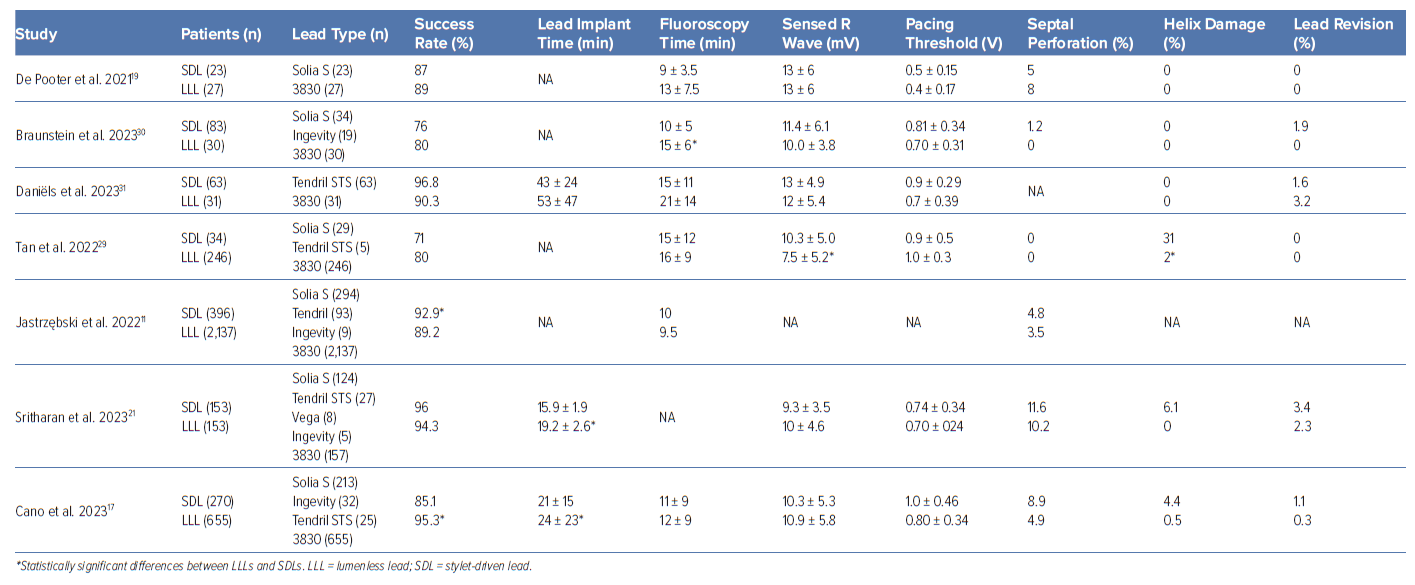
The learning curve for His bundle pacing and LBBAP has been studied in different series. For HBP using LLLs, fluoroscopy time and His bundle capture threshold decreased progressively with more experience plateauing after 30–50 cases.15 In the MELOS registry, both fluoroscopy times and V6R-wave peak time decreased over the first 110 LBBAP procedures and remained stable afterwards.11 This registry included mostly patients implanted with LLLs (1,902 patients) but also 369 patients implanted with SDLs. However, no specific information about potential differences in the learning curve between these lead types were described. In the same manner, Wang et al. reported the first 50 cases as the steepest part of the learning curve with LLLs for LBBAP, after which both implant and fluoroscopy time decreased, plateauing after 150 cases.16 Yu et al. recently evaluated the learning curve for LBBAP using SDLs in a small series of 50 patients, showing that the plateau for procedure and fluoroscopy time was reached after 24 and 25 cases, respectively.13 In a retrospective series that included 925 LBBAP implants (655 LLLs and 270 SDLs), Cano et al. found significant differences in implant success rates between LLLs and SDLs, even after the first 100 implants for each lead type (97% for LLLs versus 86% for SDLs, p=0.013) in a study carried out in two highly LBBAP-experienced centres, suggesting that even implanters with extensive previous experience using LLLs may need a specific learning curve for LBBAP using SDLs.17
Differences in Lead Design and Delivery Sheaths
Whereas several types of SDL (Solia S [Biotronik]; Tendril STS [Abbott]; Vega [MicroPort]; FINELINE Ingevity and Ingevity+ [Boston Scientific]) have been used for CSP, the largest experience in CSP has been achieved with one single type of LLL (SelectSecure 3830, Medtronic).1–12,17–21 In the perspective of CSP, important differences between SDLs and LLLs exist in terms of lead and helix design.20,22 As LLLs lack an inner lumen they tend to have thinner lead body diameters (4.1 Fr) compared to SDLs (5.6–6 Fr) which are provided with an inner lumen for stylet insertion. With the stylet inserted, SDLs tend to be stiffer compared to LLLs, which are rather floppy and tend to have better torque transfer than LLLs, but both types of leads are preferentially used with dedicated guiding sheaths when CSP is attempted.23 Larger lead body diameters might have the advantage of more grip when rotating the outer lead body (as is generally done with CSP), although the tactile feedback is also influenced by the outer insulation. Current leads have an outer insulation consisting of either polyurethane, silicone or a specific copolymer (Optim™, Abbott) whereas the inner insulation is generally made of silicone. As CSP requires rotations applied on the outer lead body, some combinations of inner and outer insultation material are more prone to wrinkling of the different layers of insulation, which is the case of the Abbott Tendril™ STS lead. Besides differences in lead body design, the uttermost important difference between LLLs and SDLs is the helix design. Most SDLs (except FINELINE) present with an extendable-retractable helix mechanism, whereas the helix of an LLL is a fixed helix design. Helix lengths of SDLs and LLLs are comparable (1.8–2 mm) and steroids are present either coated at the helix or in a capsule at the distal lead tip. Table 1 summarises the characteristics of different SDLs and LLLs.
Most leads used for CSP are accompanied with vendor-specific delivery sheaths, both for HBP and LBBAP (Figure 1).14,20 These sheaths share in common that they tend to be double curved, including a large primary curve, allowing to reach the septum, and a secondary out-of-plane curve to obtain a perpendicular position against the septum. As lead body diameters are larger for SDLs, their respective sheaths also tend to have larger sheath diameters compared to the delivery sheaths for LLLs. Larger sheath diameters might contribute to a greater stiffness of the delivery sheaths, although sheath stiffness and robustness are also determined by the braiding in the sheath material. Guiding sheaths for CSP are available in fixed and deflectable versions. While fixed and flexible sheaths can be used for HBP, fixed sheaths are preferred for LBBAP as pronounced flexible curves tend to cause friction between lead and inner sheath material, limiting deep septal lead deployment.
Differences in Implant Techniques
The differences in lead and helix design affect lead handling during implantation and might require special attention of CSP implanters.17,19–21 In general, lead and sheath positioning during HBP and LBBAP is similar for LLLs and SDLs. With SDLs, the helix needs to be extended before starting lead deployment and the stylet should be kept to the tip of the lead to add stability on the target position. If the His bundle area is going to be mapped, keeping the helix retracted until crossing the tricuspid valve may prevent helix entanglement with the tricuspid valve or the subvalvular apparatus. Both LLLs and SDLs require clockwise rotations on the outer lead body to achieve the final target side (either His or LBBA). As LLLs have a fixed helix design there is no risk of unwanted helix retraction during septal lead deployment, which is an issue with SDL. The mechanism by which the helix can retract during rotations of SDLs is due to the outer lead body slipping over the inner coil with the latter being connected with the helix.19,22 The net result is that helix retraction can occur during His or deep septal lead deployment with SDL. Screwing into the tissue with (even partially) retracted helix is not desirable as progression in the tissue will become difficult and even if successful, proper lead fixation might become impaired. To avoid helix retraction of SDLs, two strategies can be applied. The first strategy implies locking of the lead pin to avoid helix retraction. This locking of the lead pin can be achieved with stylet insertion tools, cut lead end caps or dedicated helix locking.19,22 To minimise helix retraction, locking of the helix can be further accompanied by pretensioning the inner coil and keeping that torqued inner coil tensioned during the screwing process. A second strategy is to start lead deployment with the helix extended and simply re-extend the helix if helix retraction occurs. Helix extension, by rotating the lead pin, is most often possible even at mid or deep septal lead positions. Good hints for timely diagnosis of helix retraction are either fluoroscopic landmarks or increased unipolar pacing impedance (generally above 800 Ω).
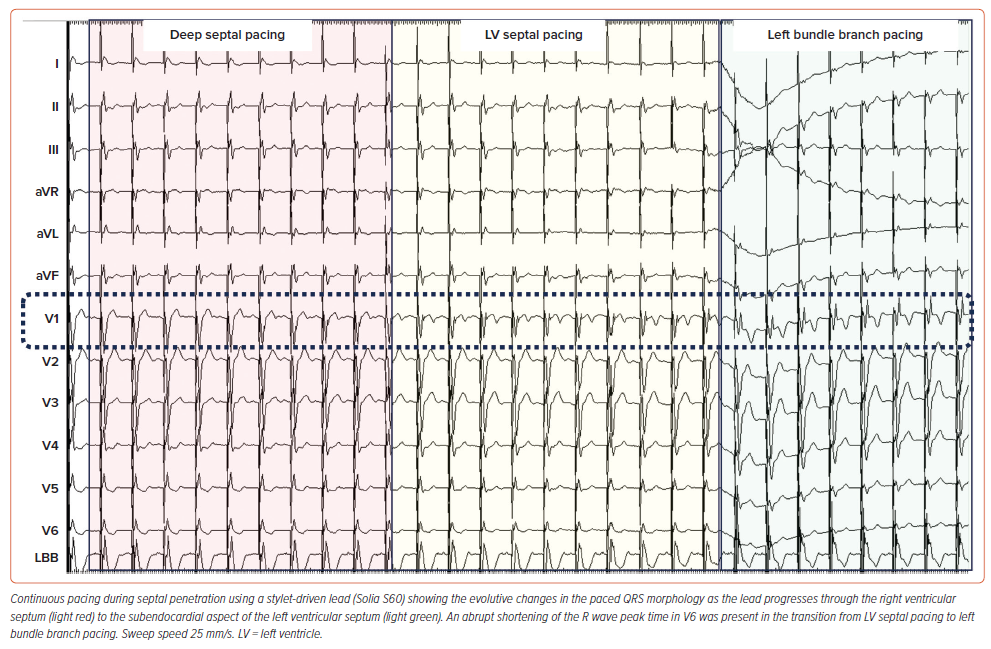
Limitations and advantages of SDLs versus LLLs have been reported previously.20 SDLs tend to be stiffer when the stylet is inserted and allow for more push on the lead. Although this might facilitate tissue penetration, it does not appear to result in more septal perforations as long as current of injury and impedance are monitored cautiously to avoid impending perforation.11,12,17,19,21,24 Besides adding stiffness to the lead body, the stylet also contributes to the torque transfer of the lead body rotations towards the helix and avoids twisting of the lead body in front of the guiding sheath.23 With SDLs, unipolar pacing can be applied on the stylet, allowing continuous pacing and impedance monitoring while easily rotating the lead body (Figure 2).22,25 However, custom-made adapters have been reported to allow for continuous monitoring with LLLs too.26 The main advantage of LLLs is the fixed helix design which does not carry the risk of unwanted helix retraction and might result in lower rates of micro-dislodgement.17,21,27 The exact mechanisms of micro-dislodgement are not fully elucidated, but it is assumed that partial helix retraction might be one of the underlying causes together with the presence of ‘drill effect’.28 It is also important to release the previous lead pretensioning before testing as some backspin may occur and may account for micro-dislodgement of SDLs. The fixed helix design of LLLs is less prone to helix damage compared to the more complex helix design of SDL, although this might also be related to the learning curve.12,29 Most cases of helix damage originate from attempts to remove an entangled or entrapped helix. Helix entanglement can occur both within the endocardial layer of the septum or the tricuspid apparatus and can be avoided by mapping the His or septum either with retracted helix or with the extended helix protected within the guiding sheath.20
How to Choose between Stylet-driven Leads and Lumenless Leads
The availability of multiple leads and sheaths from different manufacturers has expanded the implanting options. Consequently, the question arises: how does a physician determine the most suitable lead? Existing literature data reveal no discernible disparities in electrical parameters, thereby largely deferring the choice of lead to operator preference. In the absence of definitive data, the authors observe that discrepancies are more conspicuous in sheath characteristics than in leads. Notably, the availability of diverse curves enhances the feasibility of accessing different target sites and this is particularly relevant in the presence of enlarged right heart chambers. These differences are particularly prominent when targeting the atrioventricular node for HBP, wherein a higher success rate for HBP placement is noted with a shorter primary curve of the sheath, regardless of the lead used. Occasionally, the sheath may be reshaped to achieve optimal positioning as well as the stylet in SDLs. Based on our experience, in selected cases, successful procedures were achieved by transitioning from a stylet to a non-stylet-driven lead after selecting an optimal sheath.
A notable strength of SDLs lies in the rigidity of the entire system, encompassing both the sheath and the lead, theoretically allowing a higher power for lead penetration. In cases of lead dislodgement after sheath slitting, SDLs can be repositioned in right ventricle (RV) septal or apical positions without having to regain vascular access. Conversely, the major weakness in SDLs is observed in the helix, which may occasionally fail to deploy properly or retract during lead deployment manoeuvres. However, LLLs offer the advantage of extensive experience since their introduction in 2003 and thus more available data in the literature. Their thinner diameter simplifies manoeuvres primarily to rapid lead rotation rather than pushing. At present and considering the absence of randomised studies comparing the durability and long-term performance of both lead types, the final decision of whether to use an LLL or an SDL should be entirely left to the discretion and experience of individual implanters. As most implanters have previous experience with SDLs, they may find handling SDLs easier in comparison with LLLs as the system with the sheath and lead inside is stiffer and there is more grip on the lead. However, it is advisable to ultimately master both lead types because the increased experience with different tools may increase our overall implant success rate. This has been illustrated by Sritharan et al. who showed that patients with a failed implant with one lead type could be successfully implanted transitioning to the other lead type in half of the cases.21
Clinical Success Differences and Lead Performance
A few observational studies have directly compared SDLs and LLLs (Table 2).17,21,29–31 Sritharan et al. prospectively analysed a total of 306 consecutive patients undergoing LBBAP implantation at a single centre including 153 SDLs from four different manufacturers and 153 LLLs.21 No difference was found in the success rate when comparing lead type (96.0% with SDL versus 94.3% with LLL, p=0.56). Electrocardiogram acute and follow-up electrical parameters were comparable. Cano et al. compared 655 LBBAP patients using LLLs with 270 patients using SDLs and showed a significantly higher implant success with LLLs (95% versus 84%) that persisted even after excluding the learning curves for both groups (97% versus 86%, p=0.013).17 Although statistically significant differences in some electrical parameters have been described both acutely and during follow-up between SDLs and LLLs, these differences have no clinical relevance.17,32 A shorter lead implant and fluoroscopy time has been described for SDLs in comparison to LLLs in some series.17,30
Lead instability after implant represented by micro- and macro-dislodgement is another matter of concern that has been under-recognised until recently. Macro-dislodgement occurred in 8 of 147 patients (5.4%) with SDLs in a series by Sritharan et al. in comparison with 4 of 132 patients (3%) implanted with LLLs, p=0.39, with all except one being diagnosed during the first 24 hours after the implant procedure. In the same series, micro-dislodgement occurred in 9 of 147 patients with SDLs (6.1%) versus 4 of 132 with LLLs (3%), p=0.26.21 Acute micro-dislodgement occurring shortly after sheath slitting and defined by significant paced QRS morphology changes (i.e. loss of previously present ‘r’ prime in V1 or loss of left bundle branch [LBB] capture criteria) has been also described as occurring more frequently with SDLs (4.7% with SDLs versus 1.1% with LLLs, p=0.03) although lead dislodgement rates during follow-up were comparable in this series (0.9% for LLLs versus 1.5% for SDLs, p=0.489).17 In another series that included 280 patients undergoing CSP, 246 initially received LLLs and 34 SDLs with comparable implant success rates (80% for LLLs versus 79% for SDLs) but with loss of conduction system capture before discharge being more frequent with SDLs (6% versus 1%, respectively, p=0.05).29
Direct comparisons between LLLs and SDLs are scarce and imbalanced as most of the series include a significantly higher number of patients with LLLs. This disparity in the previous experience between lead types might explain some of the differences described precluding any definitive conclusion.
Data about long-term performance of LBBAP leads are also scarce. The consequences of chronic mechanical stress associated with a deep septal placement of the lead had not been studied before both LLLs and SDLs began being used for LBBAP. None of the currently used leads were initially designed for LBBAP and concerns about the risk of chronic lead fracture have arisen. Using an accelerated in vitro model of LBBAP with the 3830 lead (five applications of 20 turns followed by up to 400 million bending cycles) Zou et al. calculated an expected 10-year fracture rate of 0.02%.33 Özpak et al. recently described a 0.6% incidence of SDL lead fracture during a median follow-up of 18 months in 352 patients receiving a Solia S60 SDL with lead fracture occurring at 6 and 18 months of follow-up, respectively.34 Of note, no single case of lead fracture occurred over a median follow-up of 99 months in a retrospective cohort of 149 patients with conventional right ventricular myocardial pacing using the same lead model that served as the control group in this study. In both cases, lead fracture location was described at the intersection of tip housing and ring electrode. Although these are low numbers, uncertainties about the long-term performance of pacing leads located inside the interventricular septum for LBBAP still remain. For instance, the 0.6% of lead fracture is more than 10-fold higher than the expected incidence of lead fracture for the same lead model conventionally placed in the right ventricular myocardium according to the manufacturer product performance report. To date, only a single case of conductor fracture in an LLL lead has been described in the literature occurring 2 years after lead implant and resulting in complete loss of capture.35 The fracture was located proximal to the ring electrode and away from the lead-septum junction.
Lead extraction is another matter of concern with CSP. For LBBAP, the implications in terms of the selection of the extraction tools and clinical outcomes of an LLL or a chronically implanted lead deep inside the interventricular septum have not yet been fully evaluated. An international multicentre observational study has recently evaluated 341 patients undergoing lead extraction (224 HBP and 117 LBBAP).36 Overall, complete procedural success was 99% and clinical success 100% with mechanical tools being used in 10%, laser in 3% and femoral tools in 0.9%. Among HBP, up to 99.6% of leads were LLLs with one atrioventricular (AV) block occurring during extraction, one patient with a retained lead fragment and two patients with other lead dislodgements as procedural complications. In the 117 patients with LBBAP leads (84% LLLs, 16% SDLs) mean dwell time was 9 ± 10 months (48% with >6 months of dwell time and 12 patients with >24 months), with the longest dwell time being 35 months. Extraction success in the LBBAP subgroup was complete in 98.3% with a retained distal fragment in the remaining 1.7%. Of interest, manual traction was effective in 92% of these cases while mechanical sheaths, laser or a femoral approach was employed in 7%, 2% and 1.2%, respectively. Complications occurred in three patients (2.6%); two with a retained distal portion of an LLL in the septum and one developing severe tricuspid regurgitation after extraction of an SDL.
Complications
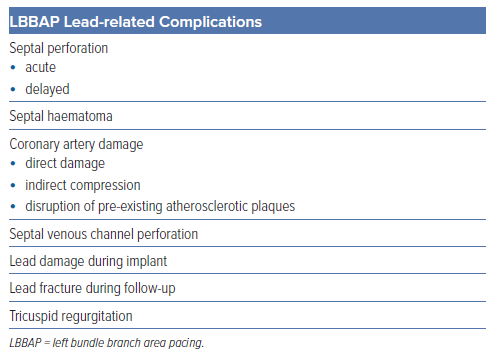
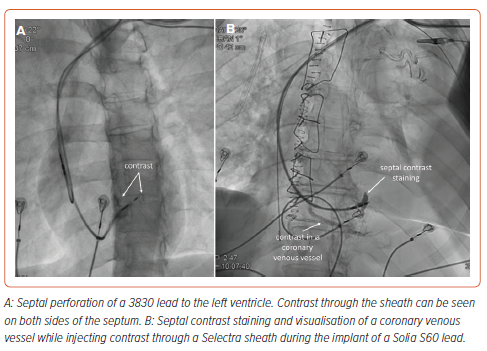
The principal complications specifically related to the transseptal route of LBBAP leads include septal perforation, septal haematoma or coronary artery complications. Other complications have been also described, including tricuspid regurgitation and septal venous channel perforation (Table 3).37,38 Among them, acute septal perforation during lead implant is the most frequently recognised but, fortunately, usually has no clinical consequences with no reported cases of persistent ventricular septal defects or any other complications related to it being described in the literature (Figure 3 and Supplementary Videos 1 and 2). Acute lead perforation is commonly resolved by lead removal and repositioning in a different septal location without precluding final implant success. Delayed septal perforation is of significant concern as it implies loss of capture and requires a re-intervention to reposition the lead. Fortunately, delayed septal perforation incidence is low, occurring in 0.08% of 2,553 patients in the MELOS registry.11
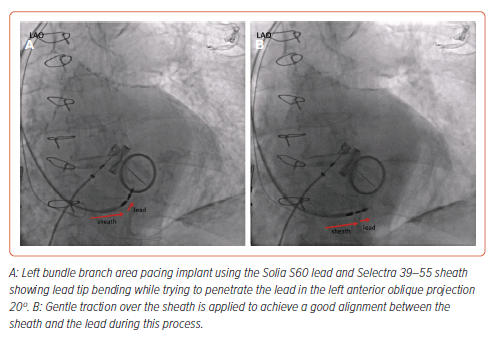
Lead damage during implant due to helix entrapment or distortion has been also described, more frequently with SDLs. Cano et al. reported 15 cases of lead damage during implant among 925 patients (1.6%), 4.4% with SDLs versus 0.5% with LLLs, p<0.001.17 In all cases the damaged lead was removed with simple traction except in one case that required a locking stylet and resulted in significant damage to the tricuspid valve with severe tricuspid regurgitation. In the same manner, helix damage due to entanglement with tissue has been described by Sritharan et al. in 10 of 164 (6.1%) patients receiving an SDL with no such cases among 157 patients in whom an LLL had been used, p=0.007.21 Tan et al. also described lead damage during implant requiring lead removal occurring more frequently with SDLs (31%) in comparison with LLLs (2%).29 Thus, special care should be taken when using SDLs to avoid this complication. Lead screwing should be stopped if lead tip bending is noticed (Figure 4) and, whenever helix entrapment is suspected, counterclockwise lead rotation without pulling back the lead should be applied before any further sheath manipulation to avoid entanglement. Entanglement may be suspected by the presence of strong torque build-up during lead rotations without significant lead progression.
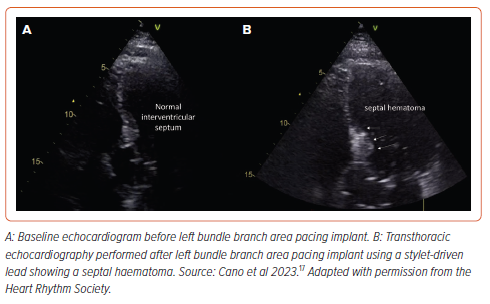
Septal haematoma is another specific complication related to the transseptal route of the pacing lead during LBBAP (Figure 5). The real incidence is not well established in the literature but a series reporting pre- and post-procedure transthoracic echocardiography showed a low incidence of this complication with only two of 925 cases (0.21%), both with SDLs and none with LLLs, resolving spontaneously without any clinical consequence.17 Other case reports in the literature also involve LLLs. Conservative management is usually effective even in the presence of giant haematomas showing spontaneous resolution after 4–6 weeks. In one case report, a coronary angiogram revealed the presence of a coronary artery to RV fistula requiring coil embolisation.39
Coronary artery complications may occur during LBBAP driven by different mechanisms including direct damage to the arteries during septal perforation, indirect compression of the vessel or disruption of pre-existing atherosclerotic plaques.40,41 This is a rare complication but has been reported in the MELOS registry, with a 0.43% incidence of acute coronary syndrome and 0.08% of coronary artery fistula.11
In the largest series reporting LBBAP outcomes using SDLs conducted in eight Belgian centres and including 353 patients, De Pooter et al. reported a 2% incidence of acute septal perforation and one case (0.28%) of late septal perforation diagnosed 3 weeks after the implant.12 Lead revision was necessary in five patients (1.4%) and one lead fracture occurred at 7 months after the implant. Septal coronary artery fistula was described in five patients (1.4%) but all of them disappeared at 3 months follow-up echocardiography. Another series comparing SDLs to LLLs showed an overall significantly higher incidence of septal perforation with SDLs (9.2% versus 4.9% for LLLs, p=0.021) but no differences were seen when comparing the last 100 patients included in each group (8% for SDLs versus 9% for LLLs), suggesting a significant influence of the learning curve on the incidence of complications.17
Future Perspectives
Both LLLs and SDLs have been shown to be safe and effective in achieving CSP. The different lead design and structure may influence the implant workflow, especially during lead penetration for LBBAP, and acute and long-term lead performance. Data show that acute implant success is comparable in most of the series as well as overall complication rates in observational studies. However, lead damage due to helix entrapment or distortion has been more frequently reported with SDLs and may be related to a different lead behaviour during lead penetration requiring special lead management and possibly with a specific learning curve even in the presence of extensive previous LBBAP experience with LLLs. Whether this phenomenon is associated with a particular SDL model is unknown and more data in this regard is desirable. The ongoing randomised study STYLE-LBBP (NCT06049992) will evaluate the acute performance of LLLs and SDLs in terms of efficacy and complications and provide new insights.
Direct comparisons are still imbalanced because LLLs have been used for CSP for a longer time, whereas SDLs have been more recently incorporated. Long-term lead performance data are still scarce with lead fracture incidence being one of the principal matters of concern, especially considering the lack of data about the influence of mechanical stress over a lead placed deep in the interventricular septum during LBBAP. The development of new implant tools, including different sheaths and lead designs, should help to overcome some of the current limitations and reduce the incidence of complications.
As an example, SDLs with a fixed and exposed helix will eliminate the undesirable spontaneous helix retraction that can occur during lead penetration which has been suggested as one of the causes of a higher incidence of micro dislodgement. In the same manner, more data about long-term CSP lead extractability with LLLs and SDLs are necessary to elucidate if there is any difference between both lead types that may favour the preferential use of one of them ahead of the other in specific settings.
Clinical Perspective
- Lumenless leads (LLLs) have been extensively used for conduction system pacing (CSP) with stylet-driven leads (SDLs) being more recently introduced.
- Lead design and structural characteristics differ between LLLs and SDLs and may influence implant workflow and lead performance both acutely and in the long term.
- Direct comparisons in terms of lead performance and clinical outcomes are scarce and imbalanced considering the more recently available experience with SDLs.
- Electrical parameters and implant success rates appear comparable, but a higher rate of lead damage has been described with SDLs, which is probably related to the implant technique.
- With current data no specific recommendation of one lead type over the other can be made and the decision should be left to the implanter’s preference and experience.