Chagas disease is an important public health problem in Latin America. Almost 25% of the population (approximately 65 million individuals) are at risk of infection and another 6 million people are affected.1 However, migration and globalisation have resulted in the increased presence of Chagas disease worldwide, particularly in the US and Europe.
Chagas disease is caused by a parasite, the flagellate Trypanosoma cruzi, which is usually transmitted by haematophagous triatominae insects (most commonly Triatoma infestans). The progression of Chagas disease can be categorised into three phases: acute, indeterminate and chronic. The acute phase occurs after the initial transmission or because of reactivation of a chronic infection in an immunosuppressed individual. Patients in the acute phase may range from completely asymptomatic to having a severe presentation (<1%), including fulminant myocarditis or meningoencephalitis. The indeterminate phase of Chagas disease is defined by the presence of infection (by serology) and absence of clinical signs or symptoms. Although most patients with Chagas disease remain in the indeterminate phase for life, 30% progress to the chronic phase several decades later. The chronic phase has several end-organ manifestations, including cardiac (new ECG abnormality or cardiomyopathy), nervous (dysautonomia) and gastrointestinal (megaoesophagus or megacolon). Dilated cardiomyopathy is the one most severe sequelae of chronic Chagas disease.2
Sudden cardiac death (SCD) is the leading cause of death in Chagas disease. Although the incidence is unknown, the estimated annual mortality rate is approximately 12,000, with the majority (55–65%) being sudden. Other causes of death in Chagas disease are heart failure (25–30%) and thromboembolic events (10–15%).3–6
SCD in Chagas disease is more common in males and occurs more frequently between the ages of 30 and 50 years.7-9 Although more common in patients with documented ventricular arrhythmias, SCD can also be the first manifestation of Chagas disease in patients with no previous symptoms or known heart failure.
The aim of this review is to provide an update on SCD in Chagas disease, examining predictors and risk stratification along with evidence on the use of drug treatment, catheter ablation, ICDs and pacemakers in people with Chagas disease.
Literature and Sources
We conducted a non-systematic review of the literature using the PubMed and SciELO databases and searching for all available references until July 2020. We also searched relevant grey literature from international and governmental organisations, including the Pan American Health Organization and the WHO. Search terms included: Chagas or chagasic; and sudden death or sudden cardiac death; ventricular arrhythmia or ventricular arrhythmias; cardiac implantable defibrillator, implantable defibrillator or defibrillator; pacemaker; or catheter ablation.
Inclusion criteria encompassed clinical trials, observational studies, case series and reviews. We excluded case reports, opinion papers and editorials. Searches were not restricted by language and the reference lists of selected articles were examined for additional citations. A total of 571 references were screened for the initial analysis of titles and abstracts by two independent investigators (RK and CY) and finally 102 references were considered relevant to be included for the review.
Mechanisms of Sudden Cardiac Death
The main accepted mechanism of SCD due to Chagas disease is VF. This is supported by the fact that Chagas disease is an arrhythmogenic condition with a high prevalence of ventricular arrhythmias, the fibrotic nature of the disease with frequent myocardial dyskinesia and/or akinesia and the reentrant mechanism of sustained ventricular tachycardia (VT) induced by programmed ventricular stimulation (PVS).10–19 Less frequently, a bradycardia (sinus node dysfunction or atrioventricular [AV] block) or pulseless electrical activity can be the cause.20 Other mechanisms are possible, such as the spontaneous ventricular rupture of an apical aneurysm.21,22
Risk Stratification
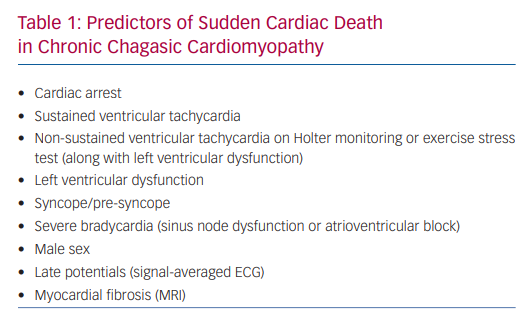
SCD in Chagas disease is more common in patients with documented ventricular arrhythmias but can also be the first manifestation in patients with no previous symptoms or known heart failure. However, most authors agree that patients in the indeterminate phase of the disease (positive serological test and normal ECG, chest X-ray and echocardiogram) carry a good prognosis with mortality rates similar to the general population.23–27 The variables identified as predictors of SCD in Chagas disease are shown in Table 1.
Spontaneous, exercise-induced or PVS-induced VT are major predictors of SCD. The survival of patients with spontaneous VT with no treatment was less than 10% at 8 years follow-up, with more than 70% of deaths occurring during the first 2 years and with 90% of deaths occurring suddenly.4 In a 2-year follow-up study, SCD was found in 16% of 44 patients with exercise-induced VT compared to none of 24 patients with no VT during exercise stress test.28 PVS-induced VT was associated with a survival of 25% at 56 months follow-up in a group of patients with non-sustained VT and mean ejection fraction of 47 ± 18% compared to a survival of 62% in a group of patients with non-inducible VT. Polymorphic VT and VF were not associated with an adverse prognosis.29,30
Non-sustained VT (NSVT), a frequent finding in chronic Chagas cardiomyopathy, is another major risk factor in predicting SCD, particularly when associated with a reduced left ventricular ejection fraction (LVEF).30–32
New York Heart Association (NYHA) functional class and left ventricular dysfunction are also important prognostic variables in chagasic patients. Survival is 97% at the 3-year follow-up for patients in NYHA Class II but only 16% for patients in NYHA Class IV. Likewise, survival at the 3-year follow-up is 100% when the LVEF is >50%, 70% when LVEF is 31–50% and only 16% when the LVEF is ≤30%.33 Recent publications have highlighted that the wall motion score index is a prognostic marker, independent of LVEF.34 Furthermore, in some instances, SCD may occur in patients with exercise-induced VT despite a relatively preserved ejection fraction.28
Pre-syncope and syncope are frequent symptoms in chronic Chagas cardiomyopathy and can be due to bradycardia or tachycardia. NSVT and bradyarrhythmias are frequent on 24-hour Holter monitoring in patients with pre-syncope or syncope (80% and 30%, respectively), and sustained VT can be induced in up to 36% of patients with syncope. Using electrophysiology studies, node dysfunction or abnormalities of the His-Purkinje conduction system were found in 40% of patients with pre-syncope or syncope.30
Complete AV block is also associated with a poor prognosis in Chagas disease. In one study of 147 patients, only 33% with no treatment survived at the 3.6-year follow-up, and most deaths were sudden.20
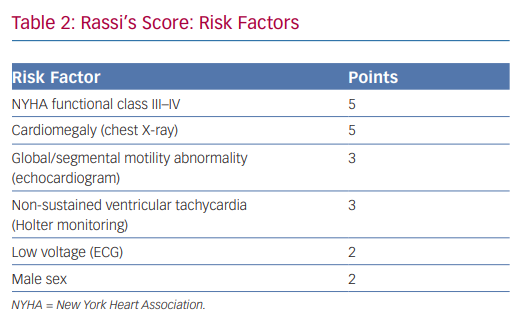
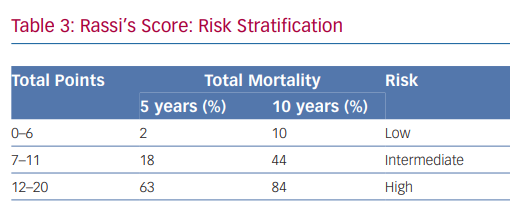
In 2006, Rassi et al. developed a risk score to predict death in Chagas heart disease.35,36 The Rassi score was developed in 424 patients with Chagas cardiomyopathy and was then validated in a separate cohort of 153 patients.37 In the initial cohort, the mean patient age was 47 years and there was a 31% mortality rate during the 7.9-year mean follow-up. Death was sudden in 62%. Multivariate analysis identified six independent predictors of mortality, and each predictor was assigned a point value (Table 2). The 5- and 10-year mortality for the low-, intermediate- and high-risk categories based on summed total points are presented in Table 3. The C statistic for the point system was 0.84 in the development cohort and 0.81 in the validation cohort.37 Further analysis of these variables demonstrated that the most consistent and strongest predictors of total mortality, SCD, or cardiovascular death were NYHA functional class III or IV, cardiomegaly on chest X-ray, left ventricular dysfunction evaluated by echocardiogram or cardiac ventriculography and NSVT on 24-hour Holter monitoring.35,38–40
More recently, myocardial fibrosis evaluated by cardiac MRI was shown to be a risk predictor of total mortality. In multivariate analysis, fibrosis (as a continuous variable) was an independent predictor of total mortality (adjusted HR 1.028; 95% CI [1.051–10.0005]; p=0.017). Each gram of additional fibrosis was associated with a 2.8% increase in mortality. In univariate analysis, a mass of 12.3 g or more (as a categorical variable) was an independent predictor of total mortality. However, it was not a predictor in the multivariate analysis.41 In addition to mortality, the presence of scar by late gadolinium enhancement is strongly associated with other major adverse outcomes, such as cardiovascular death, sustained ventricular tachycardia and cardiovascular hospitalisation.42 Moreover, myocardial delayed enhancement by MRI also quantifies myocardial fibrosis that can be detected in the early asymptomatic stages and additionally parallels well-established prognostic factors, including NYHA class, LVEF and left ventricular wall motion abnormalities.43 Furthermore, regardless of ventricular function, the degree of fibrosis seems to correlate with the presence of ventricular arrhythmias.44
Prevention of Sudden Cardiac Death
Anti-arrhythmic Drugs
Propafenone, disopyramide, mexiletine, sotalol and amiodarone are effective for ventricular arrhythmia control in chronic Chagas cardiomyopathy.45–54 However, these anti-arrhythmic drugs do not reduce mortality in clinical trials.55–57 Unlike Class I anti-arrhythmic drugs, randomised clinical trials and meta-analysis have demonstrated that amiodarone reduces mortality in patients with coronary artery disease or idiopathic dilated cardiomyopathy stratified as high risk due to complex ventricular arrhythmias and/or heart failure.58–65 Although there are no randomised clinical trials on the use of amiodarone in chagasic patients, based on extrapolation of the existing data, some experts suggest amiodarone for the treatment of chagasic patients with complex ventricular arrhythmias, particularly NSVT associated with left ventricular dysfunction.30
Leite et al. studied the effect of amiodarone on patients with chagasic cardiomyopathy and symptomatic VT. Patients were divided into three groups based on baseline electrophysiology studies. Group 1 (n=23) had no sustained VT induced, group 2 (n=45) had only tolerated sustained VT induced and group 3 (n=47) had haemodynamically unstable sustained VT induced. Total mortality at 52 ± 32 months follow-up was significantly higher in group 3 (69%; 52 ± 10.7 years, LVEF 47 ± 17%) than group 2 (22%; 52 ± 10.6 years, LVEF 49 ± 13%) and group 1 (26%; 53 ± 8.6 years, LVEF 48 ± 13%). Cardiac mortality and SCD were also higher in group 3 compared to groups 1 and 2.66 There are no data on the effect of sotalol on mortality in Chagas cardiomyopathy.
In general, heart failure due to Chagas cardiomyopathy is treated with standard pharmacological treatment for heart failure with reduced or mid-range ejection fraction, including beta blockade. Although patients with Chagas cardiomyopathy often have bradycardia that may limit their use, beta-blockers may confer a survival benefit. A subanalysis of the Repetitive Education and Monitoring for ADherence for Heart Failure (REMADHE) prospective trial – in which survival was lower in patients with Chagas heart disease as compared with other aetiologies – when only patients under beta-blockers were considered, the survival of patients with Chagas disease was similar to that of other aetiologies.67
Catheter Ablation
This technique is an alternative for persistent VT or recurrent VT when amiodarone is not tolerated or not effective. VT is inducible during an electrophysiology study in 63–95% of patients with spontaneous VT.16–18 The most common localisation of the reentrant circuits is the inferolateral basal aspect of left ventricle.68 Epicardial ablation techniques have been specifically developed to improve results in Chagas cardiomyopathy patients, in whom the reentrant circuit is generally not subendocardial.69 However, the complexity of the substrates in chagasic VT – which are frequently multiple, large and epicardial – has contributed to the relatively low success rate of this technique (approximately 60%).70
Preliminary studies on simultaneous epicardial and endocardial substrate mapping and radiofrequency catheter ablation as first-line treatment for VT and frequent ICD shocks in chronic chagasic cardiomyopathy demonstrated an 83% acute success rate, of which 78% were event-free at an average follow-up period of 10.4 months.71
Moreover, a recently published randomised clinical trial comparing efficacy and safety of endocardial versus endocardial/epicardial ablation in patients with Chagas diseases demonstrated that combining endocardial and epicardial VT catheter ablation significantly increases short- and long-term freedom from all ventricular arrhythmias, without an increase of periprocedural complication rates.72 However, the impact of catheter ablation on mortality in chagasic cardiomyopathy is still yet to be definitively determined.
ICDs
Although there are many studies showing the benefit of ICDs on secondary and primary prevention of total mortality and SCD in patients with structural heart disease, controversy persists about the efficacy in Chagas cardiomyopathy.73–80 Despite sudden death being the main cause of death in the population with Chagas disease, patients with ICDs maintain high mortality rates. The major causes of death are progression of heart failure and sudden non-arrhythmogenic death unrelated to ICDs – for example, secondary to stroke.81 Particularly in the context of Chagas cardiomyopathy, the latter is closely associated with thromboembolic events. The distinguishing hallmark of chronic Chagas cardiomyopathy is the left ventricular apical aneurysm, which predisposes not only to VT but also to thrombus formation.82 Furthermore, the progressive inflammation and atrial fibrosis due to persistent Trypanosoma cruzi infection contribute to the anatomical substrate that increases the risk of AF, which in turn, translates to an increased risk of stroke in chagasic patients.83–85
Possible other reasons for this discrepancy include the different proportion of patients on other treatments (angiotensin converting enzyme inhibitors (ACEI), beta-blockers, spironolactone, amiodarone and catheter ablation), as well as differences in device programming employed between studies.86
Indications for ICD in Chagas cardiomyopathy are based on non-randomised retrospective observational studies from tertiary centres and by data extrapolation of studies in other cardiomyopathies.26
ICDs in Secondary Prevention
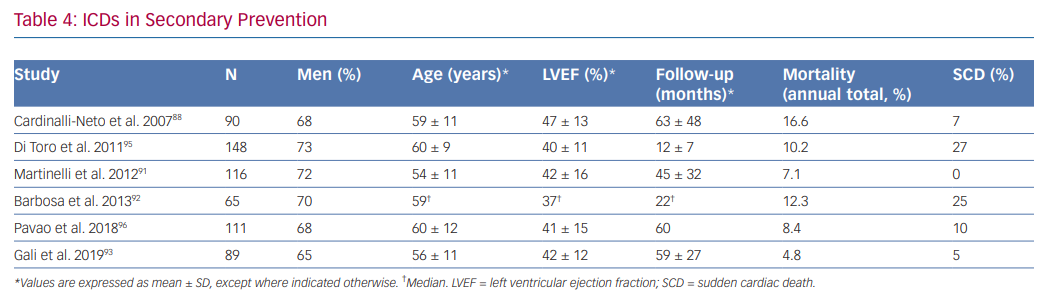
Although data derived from small, non-randomised and retrospective trials have shown that total annual mortality in chagasic patients with ICDs is low – mainly driven by a reduction of SCD – and is lower than observed in patients treated with only anti-arrhythmic drugs, there is disagreement between investigators about the benefit of ICDs in secondary prevention. Key issues include the range in total mortality rates observed in different studies, in addition to the overlapping of mortality rate between patients receiving only anti-arrhythmic drugs (5.1–11.9%) and those implanted with an ICD (4.8–16.6%; Table 4).30,66,87–94
Cardinalli-Neto et al. found a high annual total mortality (16.6%) in a group of 90 chagasic patients with ICDs (59 ± 11 years and LVEF 47 ± 13%; 28% of patients with no left ventricular dysfunction). SCD represented 7% of all deaths.88
Barbosa et al. showed a total mortality of 12.3% in 65 patients (59 years and LVEF 37%) at 266 days follow-up. SCD accounted for 25% of all deaths.92
Di Toro et al. found an annual total mortality of 10.2% in 148 patients included in a Latin American registry (60.1 ± 9.4 years and LVEF 40.1 ± 11.3%), where most patients (91.9%) had a secondary prevention indication. Age >65 years and LVEF <30% were independent predictors of mortality.95
Martinelli et al. studied a group of 116 chagasic patients with a secondary prevention indication for ICD implantation (54 ± 10.7 years and LVEF 42 ± 16%) and observed a total mortality of 7.1%. No SCD was observed. The low rate of total mortality in this study could be explained by the fact that patients with frequent episodes of VT before ICD implantation and electrical storm were treated with catheter ablation.91
In a retrospective study of 111 patients with ICDs for secondary prevention by Pavao et al. (60 ± 12 years and LVEF 41 ± 15%), the annual mortality rate was 8.4%, mostly due to refractory heart failure or non-cardiac causes. SCD only comprised of 10% of deaths. After adjusting for confounders, low LVEF, age and female gender were independently associated with death.96
Gali et al. studied a group of 89 patients (56 ± 11 years and LVEF 42 ± 12%) and did not observe benefit in a subgroup of patients >65 years old with LVEF <35% when a composite end point of total mortality or heart transplant was analysed. The annual risk of this composite end point was 20.4% in this group of patients compared to 1.4% observed in patients <65 years old with LVEF >35%.93 Although a high rate of annual appropriate therapies was observed (16%), this variable did not affect the primary end point. The low annual total mortality of 4.8% observed in this study was attributed to differences in alternative treatments, especially high rates of ACEI, beta-blocker and spironolactone use.
A recent meta-analysis suggested that an ICD does not reduce total mortality in chagasic patients compared to those treated with only amiodarone.86 Therefore, controversy about the role of an ICD in secondary prevention in chagasic patients still persists, and randomised clinical trials are needed to determine the efficacy in this group of patients.
ICDs in Primary Prevention
Although there is some evidence for ICDs in secondary prevention of SCD in Chagas cardiomyopathy, there is not enough evidence to support the indication in primary prevention.97,98 However, available evidence shows that the incidence of malignant ventricular arrhythmias and SCD in chagasic patients is higher than in other cardiomyopathies when similar degrees of left ventricular dysfunction are compared.30,92,99,100
The CHronic use of Amiodarone aGAinSt Implantable cardioverter-defibrillator therapy for primary prevention of death in patients with Chagas cardiomyopathy Study (CHAGASICS) is an on-going randomised, multicentre trial that will compare total mortality at 4.5-year follow-up in patients with a chronic chagasic cardiomyopathy, NSVT and a Rassi Score of 10 or more assigned to receive an ICD or amiodarone.101
Pacemakers
In an observational study of 147 chagasic patients with complete AV block and no anti-bradycardia therapy, the survival rates at 1, 5 and 10 years were 70%, 37% and 6%, respectively. On the contrary, in patients implanted with a VVI pacemaker, the survival rates were significantly higher (86%, 57% and 44%, respectively). SCD was observed in 87% of patients who did not receive a pacemaker compared to 67% who did.30
In a prospective cohort study (n=396), chronic Chagas cardiomyopathy patients with pacemakers had a high annual mortality rate (8.6%), despite that pacemaker-related variables were not predictors of death. The most prevalent cause of death was SCD at 34%, followed by heart failure at 32%.102
Discussion
Considering SCD as a major cause of death in advanced Chagas cardiomyopathy, many variables have been investigated to predict the risk in patients with no documented sustained ventricular arrhythmias or less advanced stages of the disease. Clinical variables related to the extent of left ventricular myocardial dysfunction (NYHA class, ECG voltage criteria, cardiomegaly and LVEF) and cardiac arrhythmias (NSVT) have been found to be the most relevant predictors.30–33 Myocardial fibrosis evaluated by MRI is a promising new risk stratification tool that could add accuracy in selecting patients at higher risk of SCD.41 The American Heart Association also recommends cardiac MRI when complex ventricular arrhythmias (especially VT) are present in patients with Chagas cardiomyopathy.26
After identifying a patient at definitive higher risk for SCD, the challenging next step is optimising evidence-based treatment options. Anti-arrhythmic drugs other than amiodarone have no demonstrated benefit in reducing mortality in chagasic patients.55,56 Although there is some evidence that amiodarone reduces mortality in patients with coronary artery disease or idiopathic dilated cardiomyopathy stratified as high risk due to complex ventricular arrhythmias and/or heart failure,58–65 there is no randomised clinical trial supporting its benefit in Chagas cardiomyopathy. Similarly, there is no randomised clinical trial on the efficacy of ICDs in secondary or primary prevention of total mortality and SCD for patients with Chagas cardiomyopathy; controversy about its role in this group of patients still persists. The indications are based on non-randomised retrospective observational studies and by extrapolation of studies in other cardiomyopathies.26
Some experts cite the high rate of appropriate ICD interventions associated with a low rate of SCD as a compelling argument for ICD implantation as standard therapy for the secondary prevention of SCD in patients with Chagas cardiomyopathy. By the same token, some authors therefore extrapolate that a randomised controlled trial comparing ICD versus amiodarone would be imprudent and unethical. However, others have speculated that the deleterious effects of ICD shocks on myocardial tissue could merely change the mode of death from arrhythmia to pump failure.94 The results of on-going clinical trials may shed light on the best treatment strategies for the prevention of SCD in patients with Chagas cardiomyopathy.101
Furthermore, approaches to further reduce ICD shocks through enhanced ICD programming strategies, broader use of amiodarone plus beta-blockers and adjunct radiofrequency catheter ablation may provide additional clinical benefit.
Conclusion
SCD is the leading cause of death in Chagas disease. Although more common in patients with documented ventricular arrhythmias, SCD can also be the first manifestation of Chagas disease in patients with no previous symptoms or known heart failure. Given the widespread global burden of Chagas disease, understanding the risk stratification and prevention of SCD in Chagas disease is of timely concern.
Clinical Perspective
- Major predictors of SCD in Chagas disease include cardiac arrest, sustained and non-sustained ventricular tachycardia, left ventricular dysfunction, syncope and bradycardia.
- Amiodarone may be beneficial for the treatment of chagasic patients with complex ventricular arrhythmias, particularly non-sustained ventricular tachycardia associated with left ventricular dysfunction.
- Catheter ablation is an alternative treatment for persistent or recurrent ventricular tachycardia. However, the complexity of the substrates in chagasic ventricular tachycardia results in a relatively low success rate.
- Controversy about the role of ICDs for primary and secondary prevention in chagasic patients persists, and randomised clinical trials are currently being conducted to determine the efficacy in this group of patients.