The use of cardiovascular implantable electronic devices (CIEDs) has increased dramatically, with approximately 1.2–1.4 million CIEDs implanted annually worldwide.1 In the US alone, there are more patients with CIEDs than registered nurses.2,3 CIEDs use leads that connect a generator to cardiac tissue to treat patients with many conditions including symptomatic bradycardia, morbid tachycardia and advanced heart failure. However, CIEDs can become infected, and leads can occasionally fail – this affects approximately 1–2 % of cases – potentially leading to adverse clinical outcomes.4 Therefore, safe, innovative techniques for lead removal are emerging to aid in the complex management of patients with CIEDs.
Once implanted, leads are held in place by scar tissue in the major veins and surrounding cardiac structures, making their withdrawal challenging. The degree of endothelial fibrosis is proportional to the length of time the lead has been implanted and the patient’s vascular inflammatory reactivity.
While open heart surgery was initially used to remove leads in the 1980s, transvenous lead extraction has evolved as the premier method over the past three decades. Compared with median sternotomy, transvenous lead extraction is an endovascular intervention more amenable for patients with several comorbidities necessitating lead removal.
This review discusses indications for transvenous lead extraction, describes each step and potential complications, and concludes by highlighting the future trends in this fascinating and ever-evolving field.
Indications for Lead Extraction
The decision to perform a lead extraction should include a consideration of many factors such as extractor and team experience, risks versus benefits, patient preference and the strength of the clinical indication for the procedure. With regards to strength of indication, the most recent Heart Rhythm Society (HRS) document divides indications into class I, IIa, IIb, or III recommendations1. Class I indications are strong and signify solid evidence or general agreement in favour of the procedure being useful and effective. Class IIa indications are considered moderate and reasonably supported by evidence, while class IIb indications are weak. The weakest strength of recommendation is class III, in which there is a general agreement that the procedure would not be useful or effective and may even be harmful.1
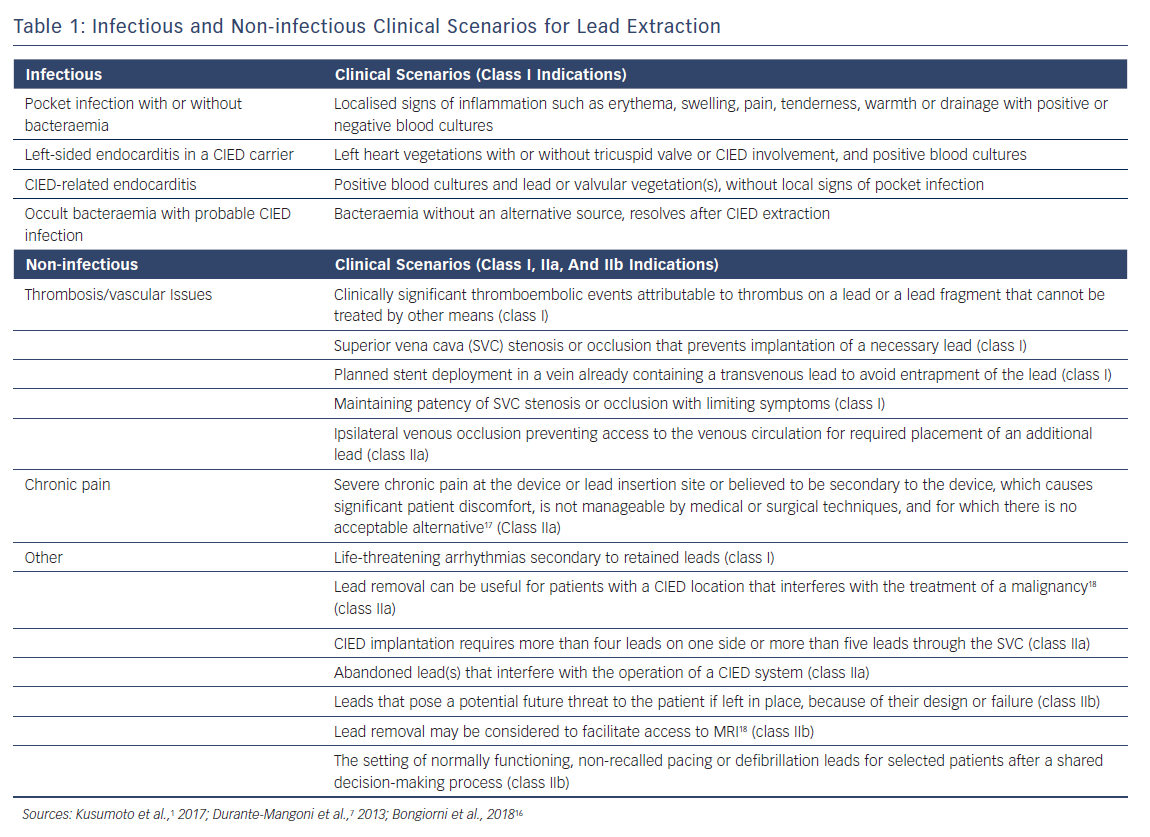
The following discussion expands on a variety of clinical scenarios in which lead extraction may be indicated. For simplicity, these have been divided into infectious (a class I recommendation) and non-infectious indications (Table 1).
Infectious
CIED infections have become increasingly prevalent because of the rise in CIED implantation, an ageing population, the existence of multiple comorbidities, and the increase in cardiac pacing centres where staff experience is inadequate.5–7 According to the recent European Lead Extraction ConTRolled registry (ELECTRa) study, infections make up 52.8 % (19.3 % systemic and 33.1 % local) of the indications for lead extractions.8 Unfortunately, infected devices are associated with significant financial burden, morbidity and mortality and require aggressive treatment.9,10 This aggressive treatment includes the complete removal of all hardware and antimicrobial therapy.
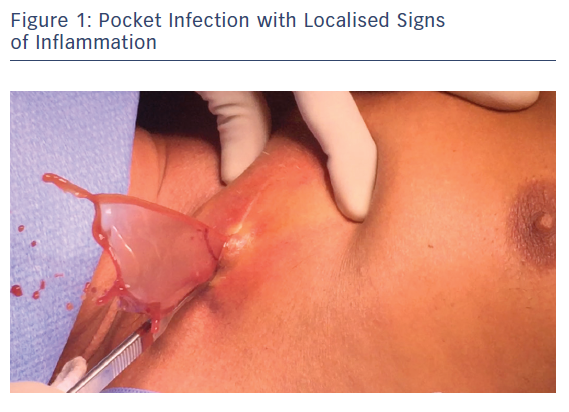
CIED infections (Table 1) have been categorised into four common clinical scenarios where complete hardware removal is required. These infections include a pocket infection with or without bacteraemia (Figure 1), left-sided endocarditis in a CIED carrier, CIED-related endocarditis and occult bacteraemia with probable CIED infection. An additional type of infection is a superficial incisional infection; here, all the hardware should not be removed because involvement is localised in the skin and subcutaneous tissue.
Symptoms of lead-associated endocarditis (LAE) may differ, according to the most recent CIED research. A study analysing patient outcomes from the Multicenter Electrophysiologic Device Cohort (MEDIC) registry determined that patients with early LAE (defined as signs and symptoms occurring within 6 months of the most recent CIED procedure) presented more frequently with signs of local pocket infection, which included erythema, pain, swelling, warmth and pus or drainage from the pocket. However, patients with late LAE (defined as signs and symptoms occurring after 6 months of the most recent CIED procedure) typically presented with signs of systemic infection, such as fever, chills, sweats and signs of sepsis.11 This discrepancy often complicates the ability to make a diagnosis. Therefore, a diligent, pre-procedural approach should be implemented to ensure the best opportunity for clinical success.
Non-infectious
The decision to perform an extraction in some non-infectious scenarios requires a complicated weighing up of the risks, benefits and long-term prognosis.
For example, if a 20-year-old patient and a 90-year-old patient present with the issue of removing an abandoned lead, the management strategies will differ, considering the shorter life expectancy in the 90-year-old patient: the 20-year-old would benefit more from an extraction (rather than lead abandonment) because of the higher incidence of major complications and increased difficulty of extraction in the future.12
In addition to lead malfunction, some important non-infectious indications for extraction include manufacturer recall, lead redundancy and a device upgrade being required because of venous occlusion.13-15 Notably, lead extractions carried out during generator change or upgrade have been reported to have fewer complications than lead-only extractions performed without a concomitant generator change.15 Table 1 sets of the most recent classification of non-infectious indications.
Facilities, Equipment and Personnel
Lead extractions are performed in operating theatres, catheterisation/electrophysiology (EP) labs and hybrid labs. A hybrid lab is a surgical suite with a movable, high-quality fluoroscopy system. The ability to provide immediate surgical intervention in cases of major complications make the operating theatre and the hybrid labs the best options to perform lead extractions. Major vascular injuries or cardiac perforations requiring surgical or endovascular intervention are rare, and these procedures may carry a higher mortality in EP laboratories than in operating theatres.12,19 Ultimately, the best location to perform lead extractions should be based on the individual facility and its team members.
It is essential that the facility provides the necessary equipment to perform lead extractions and manage complications safely.1,20,21 This should be in the room at the start of every procedure and includes equipment for transoesophageal echocardiography (TEE), fluoroscopy and arterial blood pressure monitoring, as well as a crash cart, pericardiocentesis kit, sternal saw, cardiopulmonary bypass machine, cell-saver and matched blood on standby. Most facilities have an extraction cart with all materials pertinent to the procedure.22,23
A lead extraction team typically includes a physician (who performs the extraction), a cardiothoracic surgeon (if not the primary operator), an individual in charge of providing anaesthesia support, an X-ray technician (for fluoroscopy) and assistants.23 The operator and the team must have the experience and training necessary to maximize patient safety and clinical success. The operator should have hands-on experience of a minimum of 40 lead extractions as the primary operator, with exposure to various lead types and be familiar with employing different extraction tools and approaches.1,22 The surgeon must be immediately available and be able perform an emergent thoracotomy within 5–10 minutes. Although data from a National Cardiovascular Data Registry of 11,304 ICD extractions revealed that only 0.36 % patients required urgent cardiac surgery, these emergent procedures had a 34 % mortality rate.15 Therefore, it is critical that the team is properly trained to recognise the need for surgical intervention to avoid any delays and maximise the likelihood that patients will survive any potential complications. Virtual reality training tools offering simulation have been found to enhance the skills necessary to perform extractions.24 This supplementary training method could be further implemented in the future to assess competency with the growing number of new extraction equipment.
Procedural Definitions
To allow for better discussion of the topic, specific terminologies and definitions have been established.
Lead extraction is a procedure where the removal of the lead requires equipment not typically employed during lead implantation or where at least one lead has been implanted for longer than 1 year.1,20
Lead explantation is as a procedure in which a lead is removed without specialised tools and all leads have been implanted for less than 1 year.1
In addition, clinical success for a lead extraction is defined by the removal of all targeted leads and lead material from the vascular space or retention of a small portion (<4 cm) that does not negatively affect the outcome goals of the procedure.1,16
Pre-procedure Phase
A thorough patient history should be documented including age, height, weight, current medications, New York Heart Association class and previous surgeries. There should be an evaluation of cardiac and non-cardiac conditions that could affect the procedure outcome such as diabetes, reduced left ventricular ejection fraction and out atrial fibrillation.12,16,25. Implanted devices and information about their leads (including number, location, construction, fixation type and implantation dates) should be documented. The patient’s intrinsic rhythm and dependency should be checked by CIED interrogation.26,27
Imaging
Various imaging techniques are used to determine procedural approach and the risk of complications. First, a chest X-ray should be performed for lead localisation, lead analysis and to determine the existence of calcifications. It should be noted whether the implanted leads are passively or actively fixated, given that passive fixation and dual-coil lead design may correlate with fibrous adhesions.28 The type of fixation is easily determined on chest X-ray as passively fixated leads use tines, fins or conical structures at the tip of the lead, while actively fixated leads use a corkscrew helix to screw into the myocardium.29 The X-ray is also useful to determine the presence of undocumented leads or devices that may pose issues during the extraction.
Second, a TEE is recommended for patients with suspected systemic CIED infection to determine any cardiac abnormalities including reduced ejection fraction, vegetations, tricuspid regurgitation, intracardiac shunts and pre-existing pericardial effusions.16,30–33. If large vegetations (>2.5 cm) are present, the procedure may require an alternative approach such as an open extraction.20 Because of thromboembolic risk, the presence of vegetations and their relative size should be accounted in management of antithrombotic therapy.34,35
Third, a gated cardiac CT scan is taken in some centres to check for venous stenosis or the presence of extravascular lead segments.16
Last, fluorine–18–fluorodeoxyglucose (18F–FDG) PET and CT can be used to identify infections in patients where this is suspected but not clearly evident using other imaging modalities.36,37 A recent meta-analysis with 14 studies involving 492 patients determined high sensitivity (83 %) and specificity (89 %) in the evaluation of CIED infection using PET/CT.38 The 18F–FDG PET/CT scan has been evaluated for diagnostic accuracy in other studies and its use should be considered before creating a treatment regimen where infection is suspected.39-41
Blood Tests
Before the procedure, blood samples should be collected to assess renal function, coagulation and haemoglobin, and for platelet count tests. These results should be compared with the post-procedure values.16 A minimum of two sets of blood cultures should be drawn before antibiotic therapy is started for patients with suspected CIED infection.33
For patients with infections, the firstline antibiotic should be vancomycin until the causative organism has been identified.42 The most common cause of CIED infections are Staphylococcus aureus and coagulase-negative staphylococcus.43 Nearly half of the staphylococci that cause infections are meticillin resistant which is why vancomycin is the antibiotic of choice.43
Anticoagulation
Many patients with CIEDs are prescribed oral anticoagulation or dual antiplatelet therapy. Unfortunately, lead extraction procedures carry the risk of severe and life-threatening haemorrhagic events – such as vascular tears involving the SVC, tamponade and haemothorax – and may involve thromboembolic events.20 Periprocedural management of anticoagulant therapy is essential for these patients.
Anticoagulation strategies should be considered after thromboembolic risk has been assessed. A lead extraction anticoagulation protocol should account for clinical predictors of thrombotic/thromboembolic events such as mechanical valve prostheses, out atrial fibrillation, duration of confinement to bed and length of hospitalisation.34 In the authors’ experience, continuing anticoagulation is usual practice when the patient is undergoing lead extraction.15,44,45
Extraction Approach
The extraction is conducted through the subclavian vein, the femoral vein or the internal jugular or using a combination of methods.46,47 The subclavian approach allows the complete procedure to be performed through a single incision and permits ipsilateral access to the implanting vein; it is therefore the most popular approach. Open surgical approaches are rare and usually reserved for complex and high-risk cases that preclude percutaneous methods. Such cases usually necessitate a hybrid approach that combines open heart surgery and transvenous lead extraction to remove the intracardiac and intravascular portions of the leads respectively. Recently, minimally invasive approaches have been introduced that provide an alternative to median sternotomy.48–53
Procedure Phase
First, the patient is prepped and draped in the same manner as for an open-heart procedure. Then, general anaesthesia is administered, and a TEE is carried out.1 Next, an incision is made through the original CIED implantation site to gain access to the device pocket. If there is a localised pocket infection, the pocket is debrided and microbial cultures of the pocket tissue obtained.54 If no infection is present, minimal debridement should be performed while freeing the lead from the fibrotic constraints in the pocket. The leads are then removed from the header and are dissected away from the fibrous tissue. To prevent the lead from unraveling and to apply traction across the whole length of it, the components are often secured to a lead-locking device using suture ties or a compression coil. If the lead locking device cannot be inserted, a lead extender can be used.22
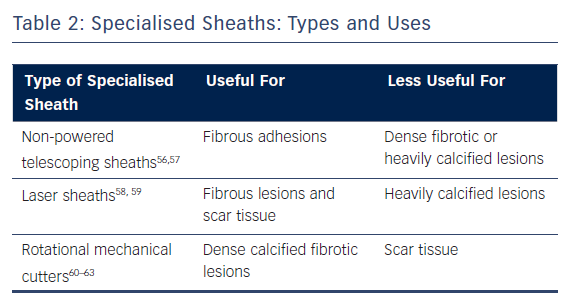
The next step depends on the degree of fibrosis, which is proportional to the age of the lead. If the lead was implanted recently, simple traction (or mild pulling with no specialised extraction tools besides a standard stylet) was found to be effective in removal of 27 % of the leads in the ELECTRa registry.8 On the other hand, if simple traction alone is unsuccessful, a specialised sheath can be used on the intravascular adhesions around the lead. The choice of sheath depends on the nature of the lesions as well as the experience, training and preference of the operator. Because of this, different sheaths may be used throughout the course of a single lead extraction depending on the circumstances (Table 2).
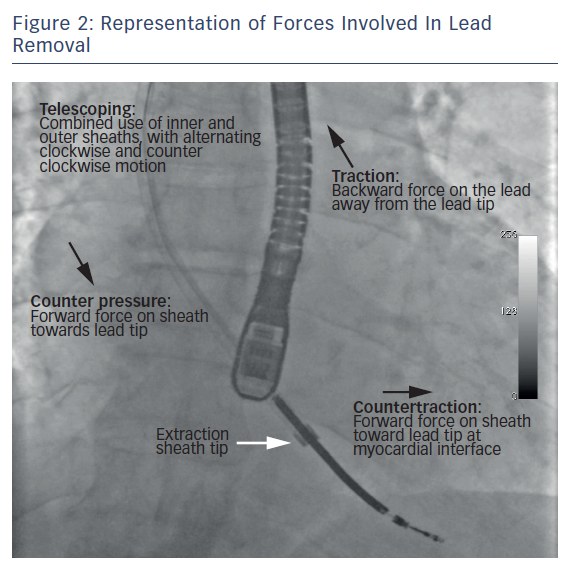
The sheath is advanced coaxially to reach the distal end of the lead (at the myocardial interface). Once the sheath is close to the myocardial interface, the lead is gently pulled in a traction-countertraction motion to release and remove the lead tip from the myocardium (Figure 2). If a new lead implant is required, a guide wire is threaded through the retained sheath to maintain venous access.22 In special circumstances such as venous occlusion and leads with minimal adhesions, femoral snaring can aid in maintaining traction while the sheath breaks through the occluded veins. In addition, a femoral approach is also useful for removing lead fragments that may break off during the extraction procedure. Furthermore, if the subclavian approach fails due to an intravascular lead break, extraction can be performed via the femoral or the internal jugular approach.55
Postprocedure Phase
After the procedure, the patient should be checked for any complications – early and late – using a chest X-ray, transthoracic echocardiogram (TTE) and physical examination.16 First, it is useful to take a chest X-ray within 24 hours of the procedure to rule out an occult haemothorax or pneumothorax. Second, a TTE after the procedure is used to assess for tricuspid valve injury, pericardial effusion and intracardial masses such as retained fragments.1 Third, physical examinations should include checking for the presence of arteriovenous fistulas from the upper arm to the subclavian area.1 Moreover, in patients with infections, additional post-procedure considerations include antibiotic selection and wound care management.1
CIED Reimplantation
After the procedure, patients are often reassessed for the clinical need for CIED reimplantation. Reimplantation may not be necessary in patients who demonstrate sufficient improvement in ejection fraction, recovery of sinus function or resolution of symptomatic bradycardia. In patients with CIED infection, reimplantation timing is not associated with risk of a second infection; second infections have been noted in patients with specific risk factors including haemodialysis, malignancy, pocket haematomas or S aureus bacteraemia.64
Complications
Overwhelmingly, transvenous lead extraction has been shown to be a safe and effective method to remove problematic leads. Poor outcomes are exceedingly rare, with several large registries reporting mortality rates from 0.2–1.2 %.1,15,19 However, serious complications that require emergent intervention may still arise in 0.2–1.8 % of cases in even the most experienced hands. There has therefore been a concerted effort to identify factors associated with complications to help clinicians stratify patients as high risk for endovascular perforations and other adverse outcomes.1,8,12,19,22,65–68 Notable risk factors identified include:
- longer lead implant duration (>6 years);8,12,19,20,66,67
- female sex;8,20,68
- low BMI or body surface area;12,66
- number of implanted leads (three or more);8,67
- infectious indication for extraction;1,22
- presence of dual-coil ICD lead;20,67
- aggressive calcified adhesions;22,65
- extravascular leads;65
- venous occlusions;65
- femoral extraction approach;8,19
- use of powered sheaths8,19,20
- renal disease (end-stage renal failure and dialysis);68
- type 2 diabetes mellitus;68
- congestive heart failure;68
- cerebrovascular disease;68
- anticoagulation or antiplatelet use;68
- chronic pulmonary disease;68
- corticosteroid use;65
- non-target lead dislodgement;15
- non-electively scheduled extraction;15,68
- low volume extraction centres;8,12,68 and
- lack of operator experience (carrying out fewer than 30 cases per year).22
Major studies have reported conflicting results in the continuing effort to identify risk factors. For instance, the ELECTRa registry of 3,510 extractions from 73 European centres concluded that high volume centres, compared with low-volume centres, were associated with higher success rates and lower all-cause complication and mortality rates.8,69 However, a study of 11,304 extractions from 762 centres across the US subsequently reported that operator annual procedure volume was not associated with a lower incidence of major complications.15 This same study found no difference in complications between dual-coil and single-coil ICD leads, and no difference in outcomes between backfilled and expanded polytetrafluoroethylene coated leads.15 That said, multiple factors may contribute to the risk of complications, including patient/lead profile and centre/operator experience. Collectively, these factors should be considered to stratify accurately for risk, prepare for complications and improve patient outcomes.
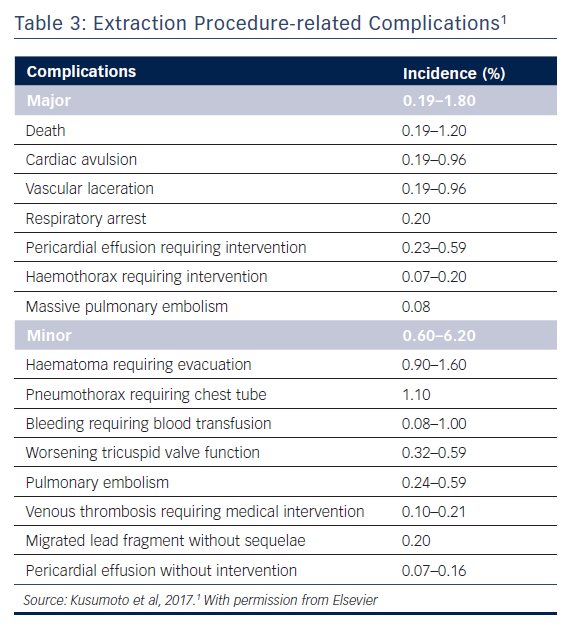
Major complications associated with lead extraction primarily arise from damage to the venous vasculature or myocardium (Table 3).1 These complications include death, vascular laceration, cardiac avulsion, pericardial tamponade, haemothorax and thromboembolic events (such as pulmonary embolism and paradoxical emboli in the presence of a patent foramen ovale or an atrial septal defect). In rare cases of rapid and massive blood loss, death is often the result. Pericardial tamponade is the most common major complication; it can be resolved if treated quickly using a sternotomy. SVC tears below the pericardial reflection may lead to pericardial effusion while tears above the reflection often result in a large haemothorax. These injuries may necessitate emergent sternotomy and surgical repair.
Minor complications include bleeding, pocket haematoma, pneumothorax necessitating chest tube placement, venous thrombosis and migrated lead fragment. Although these events are significant and require rapid intervention, they are usually not life threatening. (Table 3).
This potential for catastrophic complications underscores the importance of having a cardiac surgeon available and a competent operative team for both early recognition of injury and implementation of rapid response protocols. First, this necessitates all personnel and equipment to be available to perform an urgent sternotomy and repair, including a crash cart, sternal saw, cardiopulmonary bypass machine, cell-saver and matched blood on standby.70 Second, the operative team must be aware of the unique presentation of these injuries associated with lead extraction. When a sudden drop in blood pressure occurs, the team should immediately use fluoroscopy or TEE to identify the cause. A growing pericardial effusion, identified by the cessation of movement at the left heart border, suggests either a myocardial perforation or an SVC tear below the pericardial reflection. An empty ventricle on TEE and haemothorax on fluoroscopy suggests massive blood loss from a vascular tear above the pericardium.22 Clancy et al. demonstrated in a swine model that every second counts; a mere 2 cm tear along the SVC can rapidly haemorrhage at a rate of 500 cm3/minute, leading to complete exsanguination in less than 10 minutes.71 Finally, the nature of these injuries resembles major trauma surgery, and the operative team must be prepared to emergently manage massive bleeding to rescue the patient.
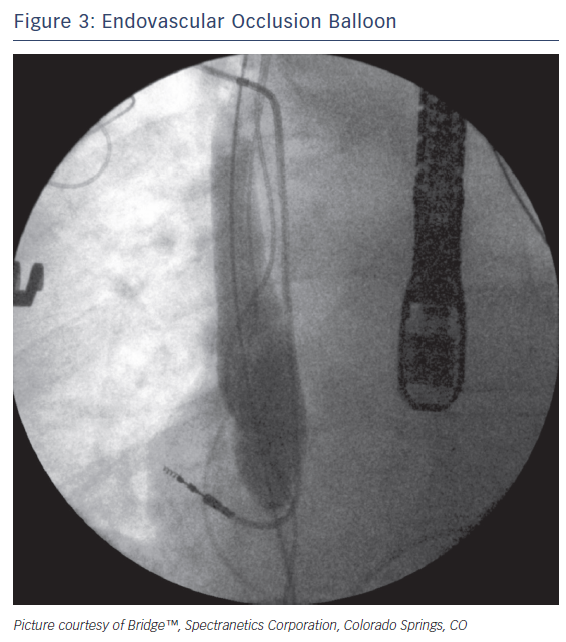
Rescue devices such as the occlusion balloon (Bridge™; Spectranetics Corporation) (Figure 3) can be rapidly deployed to help stem the loss of blood in the event of an SVC tear. The device is a compliant, endovascular balloon that occludes the SVC from the innominate veins to the right atrium and can be deployed in less than two minutes. Inflation times can be reduced to less than 15 seconds by prophylactically placing the device in the inferior vena cava of high-risk patients before extraction65. In the event of a suspected tear, the occlusion balloon can be threaded up a prepositioned wire and inflated to provide temporary haemostasis and haemodynamic stability, thereby facilitating a more controlled surgical repair. Comparative analysis of early clinical data has demonstrated that proper employment of the occlusion balloon can improve the likelihood of patients surviving these injuries.72
Future Directions
Over the past three decades, the rise in CIED implantation has been paralleled by a remarkable increase in lead extractions. As indications for therapy expand and patients with CIEDs live longer, it is likely that demand for lead extraction will only continue to grow.73 Today, methods to reduce the number of extracted leads are being explored, whether by investigating novel infection control strategies or refining alternative device therapies to transvenous systems. Additionally, recent advances in cardiac imaging modalities hold promise in making lead extraction safer.74 Above all, as lead extraction becomes safer and easier to perform, it will likely become accessible to a wider variety of clinicians and patients.
Prevention of Infection
Given that a substantial portion of lead extractions are indicated because of infection, methods to reduce rates of infection are being explored. A promising technique under study is the use of an antibiotic-eluting mesh (TYRX™ Anti-bacterial Envelope; Medtronic plc) to reduce CIED infections in high-risk patients.33,75 This bio-absorbable mesh is placed in the CIED pocket at the time of implantation and releases minocycline and rifampicin for a 7-day period. Meta-analyses have revealed significant reductions in CIED infections, and cost-effectiveness analysis has shown a reduction in healthcare resource utilisation.76,77 Over the long term, randomised controlled studies, such as the Worldwide Randomized Antibiotic Envelope Infection Prevention Trial (WRAP–IT), are in progress.78
Another important area of investigation is the use of perioperative antibiotics after CIED implantation. Currently, no guideline recommendations support the use of post-procedural antibiotic prophylaxis.79–81 A 2017 HRS survey suggested that real-world prescribing patterns vary considerably, and that post-procedural antibiotics are administered after 22–50 % of CIED surgeries.82 Moreover, the recent Prevention of Arrhythmia Device Infection Trial (PADIT), involving 19,603 patients in Canada, found that increased postoperative antibiotics after CIED implantation had no substantial effect on infections.83,84 Future analyses may help identify effective post-procedure prophylactic antibiotics strategies.
Leadless Alternatives
While transvenous pacing and defibrillating systems remain the premier strategy for treating cardiac conduction abnormalities, a few emerging device therapies may mitigate the rise in leads requiring extraction.
Leadless pacemakers (Micra™ Transcatheter Pacing System, Medtronic plc) and subcutaneous ICD systems (S–ICDTM System, Boston Scientific Corp) are attractive alternatives that supplant the need for transvenous leads altogether.85 Several large, multicentre trials for both modalities have demonstrated consistently high implant success rates and lower complication rates than conventional systems.86–92 Moreover, recent advances in operator experience, preparation and implantation techniques have led to further improvements in infection rates and wider use around the world.75,93–95
Currently, leadless pacemaker and subcutaneous ICD systems are limited to a few clinical indications. As these emerging technologies continue to develop, the leadless pacemaker and the S–ICD could play a more prominent role in the management of cardiac arrhythmias.
Advances in Imaging
Novel imaging modalities have the potential to make lead extraction even safer through better preprocedural planning and intraoperative navigation. The use of three-dimensional (3D) reconstruction of gated cardiac CT provides an unparalleled ability to visualize the CIED system in relation to intravascular and intracardiac structures.
Colour 3D Doppler echocardiography of the SVC was used by a team at Drexel University to predict lead fibrosis. This demonstrated the feasibility of a low-cost, noninvasive screening method to predict whether complex procedures would be needed.96
These modalities have enabled lead extractors to better risk stratify patients and prepare for otherwise unforeseen problems.
Additionally, recent advancements in 3D imaging technology (CartoSound™, Biosense Webster Inc) have allowed for real-time assessment of binding sites during transvenous lead extraction97. By integrating real-time, two-dimensional intracardiac echocardiography into the Carto® electroanatomic mapping system environment (Biosense Webster Inc), Nguyen et al. demonstrated that this novel imaging modality yielded better visualization of binding site volume and morphology than fluoroscopy.98 Moreover, this resulted in a significantly improved procedural success rate and reductions in complications, procedural times and radiation exposure.
Future studies are needed to continue evaluating these technologies and their integration before they can be adopted into routine clinical practice.
Prospective Innovation
The future of lead extraction will likely include more intuitive, effective tools to break adhesions and safely extract leads. We predict these tools will be simpler to use and reduce the steep learning curve currently required to gain competence in lead extraction. For example, these novel technologies may be completely different from today’s laser and mechanical techniques and may include expanding balloon or mechanical vibratory sheaths that break adhesions with ease.
As the field of lead extraction and management continues to evolve, efforts should be made to increase the use of lead extraction. Even with current guidelines, lead extractions are not carried out as much as they could be, as one third of patients with device infections do not receive the proper lead removal therapy and only 15 % of patients with abandoned leads have these extracted in the US.98,99
Ultimately, the goal of these new technologies is to reduce the burden of CIED complications and ensure all patients receive the appropriate intervention. To attain this vision, practitioners must remain committed to fostering a culture of both steadfast innovation and collaboration, while ensuring that safety and efficacy remain at the forefront of this exciting arena in medicine.
Clinical Perspective
- Multicenter, real-world registries have demonstrated that lead extraction is a safe and effective procedure to address problematic cardiac implantable electronic device leads.
- The decision to perform a lead extraction should consider the updated guidelines from both Europe and the US, extractor and team experience, risks versus benefits and patient preference.
- Transvenous lead extraction approach and risk of complications may be determined using imaging modalities, such as chest x-ray, transesophageal echocardiogram, gated cardiac CT and fluorine–18-fluorodeoxyglucose (18F–FDG) PET and CT.
- Published clinical registries for lead extraction have offered insight into potential complications and strategies to improve patient safety.
- Recent advances regarding infection control, imaging, and equipment offer promise of increasingly safer procedures and better outcomes.