The use of adenosine in cardiology is ubiquitous. From arrhythmia to coronary intervention to cardiac imaging, adenosine is an essential part of everyday practice because of its widespread effects on electrophysiology and the coronary vasculature. Electrophysiologists will be most familiar with adenosine for its use in terminating supraventricular tachycardias (SVTs) that are dependent on the atrioventricular node (AVN) and in unmasking underlying rhythms.
Decades of clinical experience in the use of adenosine in SVT have refined its clinical applications as reflected in the 2019 update of European Society of Cardiology guidelines for this condition.1 The role of adenosine in disorders encountered in cardiac electrophysiology is set to expand as patients increasingly survive ischaemic heart disease, populations become older and the burden of metabolic syndrome manifests. Adenosine signalling also has roles in atrial flutter, AF and ventricular arrhythmia. New research strongly implicates adenosine in other conditions associated with cardiac consequences, such as obesity, diabetes and pulmonary disease. This article provides an updated and encompassing review of the role of exogenous and endogenous adenosine on cardiac electrophysiology, and provides insights into its potential future therapeutic uses.2,3
Adenosine Discovery and Early Characterisation
Sir Alan Nigel Drury and Sir Albert Szent-Györgyi (Figure 1), who worked at the University of Cambridge in the departments of pathology and biochemistry respectively, are widely acknowledged as being the first to identify the actions of adenosine on the heart in 1929.4 In the same year this work was published, Karl Lohmann was accredited with the discovery of adenosine triphosphate (ATP); these combined works heralded the beginnings of purinergic science.5 Szent-Györgyi later received the Nobel Prize in 1937 for his work on vitamin C and components of the citric acid cycle. Drury, who had previously worked closely with ECG pioneer Sir Thomas Lewis, had been responsible for seminal observations on the re-entrant mechanism of atrial flutter, vagal stimulation and the mechanism of quinidine in AF.6–9
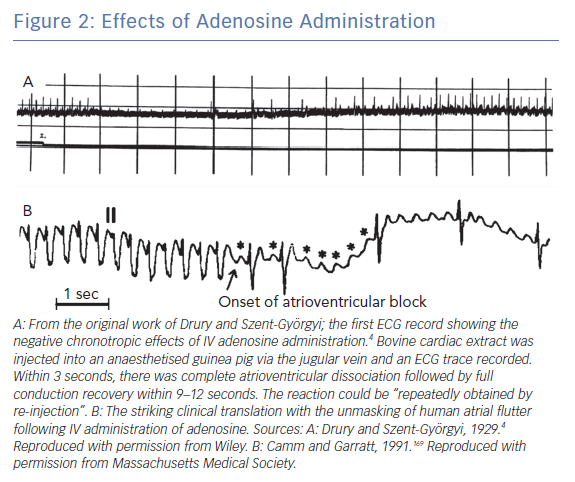
Drury and Szent-Györgyi found that injecting extracts from bullock heart into small animals resulted in transient bradycardia, which was recorded using ECGs (Figure 2A). This was not antagonised by atropine, suggesting a mechanism independent of vagal stimulation. The extract was purified and shown to be composed of adenine, a pentose sugar and phosphoric acid, and was chemically similar to adenosine monophosphate (AMP). Adenosine and adenosine diphosphate (ADP) had similar cardiac effects, whereas other nucleoside products, such as guanosine, did not. The mechanism of the bradycardia was high-grade atrioventricular (AV) block, although sinus bradycardia was observed at higher doses. The effect typically occurred 10–12 seconds following administration, with the maximal effect at 15–30 seconds. Adenosine was also shown to reduce the refractory period and augment conduction velocity during pacing at high heart rates. They hypothesised a role for adenosine in terminating atrial flutter and AF, attributed to its effect on the atrial refractory period. The translation of adenosine use from animal studies into humans was swift, with broadly similar results found a year later.10
Pharmacology of Adenosine
Adenosine is an endogenous purine formed by adenine and D-ribose produced from the degradation of ATP, ADP and AMP by ectoenzymes (predominantly CD39 and CD73) which is a process universal to most mammalian cells.11 The production of adenosine is widely employed in cellular signalling as an indication of ATP turnover. Extracellular adenosine levels may be elevated by additional release from cardiomyocytes, platelets, endothelium and nerves induced by ischaemia/hypoxia, catecholamines and calcium.12,13
There are four G-protein coupled adenosine receptor subtypes present in cardiac tissue: A1 (Galphai), A2A(Galphas), A2B(Galphas) and A3(Galphai), which critically affect three main aspects of cardiac physiology. First, adenosine induces coronary vasodilatation, increasing blood flow according to tissue demand.14,15 This is mediated by A2 receptor signalling, especially A2A, with high potency due to large receptor reserves.16,17 Second, adenosine has negative inotropic effects via antagonism of sympathetic pathways.18,19 Third, adenosine exerts major electrophysiological effects, predominantly mediated by the A1 receptors with AMP and adenosine being 10-fold more efficacious in direct actions on downstream currents than ATP in pharmacological studies.20,21 Abrogation of electrophysiological effects by inhibiting conversion of AMP to adenosine or accelerating the metabolism of adenosine to inosine suggest that adenosine is the primary mediator of endogenous signalling at the A1 receptor.21
The cardiac expression of A1 receptors and associated G-protein-coupled, inward-rectifying potassium (GIRK) channels is concentrated in the right atrium; this is three times higher than it is in the left atrium, especially in superolateral regions. This regional heterogeneity in expression results in greater reduction in right atrial action potential duration (APD) in response to adenosine.22
Adenosine has a short half-life of less than 10 seconds, typically being cleared from the plasma within 30 seconds either by adenosine deaminase, which is present in erythrocytes and vascular endothelium, or by phosphorylation to AMP. A short half-life, as in many applications, can be advantageous because effects are short lived, which limits the potential for adverse events. However, this means both direct oral administration and long-term therapy are precluded. It can also result in drug delivery issues because a peripherally administered adenosine bolus is subject to significant dispersion and longer transit times. This allows greater metabolism and results in higher and more variable dosages. Therefore, it is advocated that adenosine should be delivered either via a central line or a large-bore cannula placed, for example, in the antecubital fossa.1,23
Methylxanthines, such as caffeine and theophylline, are adenosine antagonists, reducing its effect. Caffeine ingested within 4–6 hours, but not 6–8 hours, may mean a higher dose is required.24 Dipyridamole, an antiplatelet and vasodilatory agent, accentuates the actions of adenosine by inhibiting metabolism by adenosine deaminase; it does this both directly and, more importantly, indirectly by preventing erythrocyte uptake of adenosine where it is subsequently deaminated by inhibition of adenosine transporters.25–27
Electrophysiological Effects of Adenosine
Adenosine exerts its electrophysiological effects via the A1 receptor, which couples with GIRK channels, similar to the mechanism of the muscarinic acetylcholine receptor.28 There may also be interactions with the KATP channel.29 GIRK channels are responsible for the IKAdo current, which causes membrane hyperpolarisation. In addition, the Galphai subunit of the A1 receptor inhibits cyclic AMP (cAMP) production, therefore reducing the beta1-adrenoreceptor response to catecholamines and reducing L-type Ca2+ channel currents, again paralleling muscarinic pathways.30 Increased IKAdo results in a reduced APD and therefore a shorter refractory period.21 Because of a greater receptor reserve and therefore downstream amplifying signalling pathways, adenosine is 11 times more efficacious at inhibiting beta1-adrenoreceptor response than activating IKAdo.31,32
Regional variations in cardiac adenosine response are dependent on the density of adenosine receptors as well as site-specific variations in function, other receptor expression and ion channel distributions. Membrane hyperpolarisation and reduction in cAMP levels in the sinoatrial node result in negative chronotropy via reduced pacemaker currents (If), with effects being more pronounced in conditions of higher sympathetic drive.33,34 Additional negative chronotropic effects might be mediated by reductions in cytosolic Ca2+ release and subsequent decreases in repolarising K+ channels that regulate automaticity.35
In the AVN, which shows high A1 receptor density in the nodal region, the inhibitory effects of adenosine result in negative dromotropy and reduced gradient of the cardiac upstroke.20 In atrial myocardium, shortening of the APD and reduced cAMP result in reduced inotropy and refractory period.36 His-Purkinje automaticity is reduced by adenosine in the basal state but more markedly in the presence of catecholamines.37,38 The ventricular myocardium is relatively unaffected by adenosine at rest; however, ventricular APD and inotropy are reduced by adenosine in the presence of increased sympathetic tone.39
The effects of ATP are broadly similar to those of adenosine, reflecting the rapid metabolism of ATP to adenosine that likely exerts direct cardiac actions with high potency at a molecular level.21 However, ATP has the additional effects of increasing vagal tone via P2X receptor stimulation, which potentially explains the more potent in vivo bradycardic effects of ATP on a mole-for-mole basis.40,41
Role of Adenosine in Supraventricular Tachycardia
Presentation with SVT in the emergency department is common and accounts for significant repeat attendance. SVT usually results in unpleasant symptoms of palpitations and pre-syncope, but is rarely life-threatening in isolation and often spontaneously self-terminates. Guideline-based management of SVT suggests a trial of vagal manoeuvres in the first instance, because these may be effective in up to 43% of patients, particularly modified Valsalva techniques.1,42 Valsalva techniques may be contraindicated in recent MI or stroke, glaucoma, retinopathy, carotid artery stenosis, aortic stenosis or the third trimester of pregnancy.43 Adenosine is then recommended if vagal manoeuvres fail or are inappropriate, and is successful in approximately 90% of cases. Direct current cardioversion is indicated if the patient is haemodynamically compromised.
Atrioventricular Node-dependent Supraventricular Tachycardia
The first published reports of purinergic compounds employed to terminate tachycardias were in 1955 and used ATP.44 However, purinergic compounds did not enter widespread clinical use until the 1980s, when DiMarco et al. first demonstrated the clinical efficacy of adenosine and Greco et al. showed the efficacy of ATP in the termination of SVT.45–47 These case series showed adenosine to be efficacious in SVTs where the re-entrant circuit involved the AVN, particularly atrioventricular re-entrant tachycardia and atrioventricular node re-entrant tachycardia.
Adenosine and ATP are thought to have similar efficacy at terminating SVT.48 Side-effects were noted to be minor and transient, but doses required were variable. This was accounted for by rapid clearance, variable rates of injection through different cannula sizes and the patient’s degree of intrinsic sympathetic tone. In SVTs that were not AVN-dependent, adenosine was established to be useful in unmasking underlying rhythms when these were obscured by rapid ventricular capture. Adenosine can also be used off label to elucidate dual AVN physiology or concealed accessory pathways to facilitate ablation procedures.49,50
While adenosine is effective at terminating AVN-dependent SVT, other pharmacological agents are also useful.1 Calcium channel antagonists (CCAs), of which verapamil is the best studied, are often reluctantly prescribed by general physicians in the emergency department. The potentially mortal risks of verapamil in patients with ventricular arrhythmia misdiagnosed as SVT have earned it the unfortunate epithet of ‘verapakill’. However, when used appropriately in SVT, CCAs are as clinically effective as adenosine.51
The time to cardioversion with CCAs is slightly longer and the risk of hypotension is slightly greater, although the latter is rare and is mitigated by slower infusions.42 The rates of minor side-effects were higher with adenosine. CCAs may therefore be a good alternative to adenosine where patients are haemodynamically stable and in whom adenosine is contraindicated. The longer half-life of verapamil may also make it more cost effective as a single administration than repeated doses of adenosine, and it may be more suitable for longer-term administration. However, CCAs should be avoided in heart failure, concomitant beta-blocker use and in broad complex tachycardias that might represent ventricular tachycardia (VT).
Both adenosine and CCAs are superior to beta-blockers in SVT termination, and beta-blockers are associated with an increased risk of hypotension, which makes them a third-line therapy.1 The exception to this is in pregnancy, where adenosine (which does not cross the placenta) is used as first-line therapy and beta-blockers might be considered before CCAs because the latter pose an increased risk to the fetus.42
Attempts to reduce the burden of SVTs on emergency departments have included examining paramedic-led adenosine administration in the community when the patient has a narrow complex tachycardia without a history of structural or ischaemic heart disease.52 Successful adenosine cardioversion at the scene of presentation allows immediate discharge of certain patients with follow-up arranged by the primary care physician. In this study, 81% of adenosine-treated patients cardioverted to sinus rhythm and 77% of these could be directly discharged, accounting for more than half of the patients treated.
Paramedic-led care was associated with faster treatment times, higher patient satisfaction and a significant cost reduction of about one third. Follow-up rates were similar for paramedic and hospital treated patients. This is important because, while adenosine is effective in the termination of AVN-dependent SVT in the short term, ablation should be considered as the gold-standard, long-term treatment.1 Healthcare use analysis shows that patients seen by an electrophysiologist have a reduced need for emergency services for future episodes and that 78% of the patients referred to an electrophysiologist were subsequently ablated, with a 91% success rate.53 Adenosine sensitivity identified patients who were more likely to respond to ablation therapy.
SVTs are the most commonly encountered arrhythmias in children and may precipitate heart failure in infants.54 Vagal manoeuvres terminate 25% of paediatric cases, with adenosine success in 79% of cases.55 SVT refractory to adenosine is encountered in 15% and is more likely in younger infants; higher doses may be required in these circumstances.54 The reasons for this are not clear but contributors might include the smaller calibre of paediatric cannulae, relative AVN insensitivity or late presentation after which decompensation has already occurred. Verapamil is contraindicated in infants, making adenosine the preferred agent, although digoxin, propranolol, amiodarone and procainamide have been used.
Atrioventricular Node-independent Supraventricular Tachycardia
The circuit in atrial flutter is not AVN dependent so adenosine will not reliably terminate this rhythm. However, the dromotropic effect of adenosine results in ventricular slowing so it is frequently used in the emergency department to evaluate the underlying rhythm in narrow complex tachycardia (Figure 2B). While this is perceived as a low-risk manoeuvre, some case reports suggest haemodynamic instability can occasionally be induced by conversion to 1:1 AVN conduction, induction of AF or increased catecholaminergic drive with paradoxical worsening of tachycardia.56,57 It is therefore advisable that adenosine be avoided if the underlying rhythm is clear from the 12-lead ECG without need for unmasking manoeuvres.58 In the context of a broad complex tachycardia, where atrial flutter with aberrant conduction is suspected, adenosine is safer to use than a CCA.59
Several mechanistic studies suggest that atrial flutter is critically dependent on antecedent AF to produce a line of block between the superior and inferior vena cava, allowing re-entry around the cavotricuspid isthmus (CTI).60,61 This is supported by the results of the Impact of Different Ablation Approaches on Outcome In Coexistent Atrial Fibrillation and Flutter (APPROVAL) study, where ablation outcomes were improved by concomitant flutter and AF ablations, compared to flutter ablation alone. Animal models investigating this mechanism have shown that purine administration may reduce the refractory period of this line of block, preventing it from being sustained and thus converting flutter to AF.62 The implications of this are important for unmasking the underlying rhythm in a narrow, complex tachycardia because adenosine might convert an underlying flutter into AF, therefore misleading the interpretation of the culprit rhythm.
Adenosine has been advocated in ablation procedures for the assessment of dormant connection. Injury currents set up as a result of ablation result in membrane depolarisation, which in turn inactivates voltage-gated Na+ channels preventing initiation of excitation. Over time, these injury currents dissipate and the membrane potential may recover, allowing previously isolated regions to propagate. Adenosine transiently hyperpolarises injured regions, which may become electrically excitable following a recovery period, thus unmasking dormant connections.63
Some clinical studies demonstrate an increased detection of dormant connections of the pulmonary veins during AF ablation following adenosine testing, while others have not shown long-term improvements in outcome.64–66 This discrepancy might be due to differences in study protocol.63 Adenosine has also been advocated in CTI-dependent atrial flutter ablations to assess for dormant connections.67,68 A negative adenosine provocation test with bi-directional block after CTI ablation is strongly predictive that reconnection will not occur and may be used to reduce procedure duration by decreasing assessment time for reconnection.69
Role of Adenosine in Other Electrical Cardiac Pathology
Ventricular Arrhythmia
In contrast to the sinoatrial node, AVN and atrial structures, previous animal studies have suggested that the ventricular myocardium has negligible GIRK channels, and therefore the direct electrophysiological effects of adenosine in reducing refractory period are absent.39,70 More recent human studies, however, have demonstrated GIRK expression in the ventricle at approximately half the level of the atrium and this is involved in ventricular repolarisation, including in response to adenosine.71,72 Adenosine exerts indirect effects on ventricular myocytes via inhibition of cAMP and therefore attenuation of sympathetic actions.73 Paradoxically, adenosine may also increase sympathetic drive at the neuronal level via A2-mediated chemoreceptor pathways, though cellular mediated mechanisms are usually dominant.74
Junctional ectopic tachycardias arising from the His-Purkinje system or ventricular myocardium result from either abnormal automaticity or triggered activity, which are often catecholamine driven. Adenosine has been used to differentiate between abnormal automaticity, which is adenosine insensitive, and triggered activity, which is adenosine sensitive.75
There may be a role for adenosine in distinguishing VT whose origin is triggered activity from delayed afterdepolarisations (DADs). DADs are reported to arise when there is Ca2+ overload in the cardiomyocyte that activates the electrogenic Na+/Ca2+ exchanger. Adenosine has been shown to abolish DADs by decreasing Ca2+ influx, thereby reducing triggered activity that might be stimulated by catecholaminergic activity.76
Sick Sinus Syndrome
Some have suggested that sick sinus syndrome might be an adenosine-mediated disease, given its cardioinhibitory effects.77 However, the adenosine antagonist theophylline does not improve sinus node function, which suggests it is not a central mediator in this condition.78 Patients with sick sinus syndrome may have an exaggerated response to adenosine, particularly when challenged in the context of syncope or presyncope, possibly suggesting a role for adenosine as a non-invasive test.79 This may result from an increased expression of adenosine receptors, particularly in the elderly. Similar results have been found for ATP administration.80,81 The antiplatelet agent ticagrelor has been implicated in sinus node dysfunction and ventricular pauses, potentially via increased production of adenosine.82
Syncope
Neurally mediated syncope is thought to arise from paradoxical cardioinhibitory reflexes secondary to reductions in preload. Adenosine may be useful in the diagnosis of neurally mediated and unexplained syncope in combination with tilt table testing, particularly in the under 40s, by identifying patients susceptible to transient AV block.83–85
Pulmonary embolism results in syncope in approximately 10% of cases and may represent a significant proportion of syncope presentations to the emergency department.86,87 Purinergic signalling resulting from the release of ATP by activated platelets following pulmonary embolism has been suggested to result in transient bradycardia and syncope in these cases.88
Role of Adenosine in Other Pathology Related to Arrhythmia
Obesity and Diabetes
The prevalence of obesity has doubled since 1980, with current estimates suggesting that one in three adults is now obese, which has significant costs to healthcare services and the wider economy.89,90 Obesity is an independent risk factor for the development of AF with a 3–7% increased risk of AF per unit increase in BMI.91 Obesity also confers an increased risk of sudden cardiac death (SCD) from ventricular arrhythmia, particularly in middle-aged people and in patients with ischaemic cardiomyopathy.92–94
Suggested mechanisms proposed for increased arrhythmogenicity in obesity include increased pro-inflammatory cytokines, oxidative stress and fibrosis. Changes in electrical properties of the myocardium are also observed with shorter atrial and pulmonary vein refractory periods, impaired repolarisation in the ventricles and conduction abnormalities.95–97
Adenosine shows generally protective actions in the pathophysiology of obesity, the complex metabolic pathways of which have been extensively reviewed.98 Plasma adenosine concentrations are increased in obesity and are typically 1.5 times higher than in lean individuals; tissue levels can be higher still.99 Activation of A1 receptors facilitate insulin-dependent glucose uptake and reduces obesity-related systemic insulin resistance.100,101 Activation of A1 receptors increases plasma leptin and reduces adiponectin levels, both of which would have anti-obesogenic effects.102,103 Activation of the A2B receptors results in the secretion of anti-inflammatory cytokines such as IL–10 and IL–4.104 No studies directly link obesity, adenosine and arrhythmia; however, common pathophysiological pathways are involved. Adenosine may therefore be a significant area for future research and therapy in obesity-related arrhythmia.
Heart Failure
Worldwide, 26 million people have heart failure and estimates predict the prevalence of heart failure will have risen by 46% by 2030 in the US.105,106 The prognosis of heart failure remains worse than that for most cancers and a major contributor is a markedly increased risk of SCD, presumed secondary to ventricular arrhythmia.107 The proportion of deaths related to SCD ranges between 33% and 64%.108 Patients with heart failure also have increased risks of both SVTs and AF, which increase morbidity and mortality.13 Catheter ablation of AF in patients with heart failure may reduce mortality.109
Disruption of cardiac energetics is well established in the pathophysiology of heart failure, particularly a reduced utilisation of free fatty acids in preference to glucose as a metabolic substrate, mitochondrial dysfunction and reduced intracellular ATP levels.110–112 In later stages, insulin resistance might be responsible for reducing glucose supply to the failing heart, further switching metabolism to ketones.113
Purinergic receptors are upregulated in heart failure and plasma levels of adenosine also increase.114,115 Therapy with adenosine may have a cardioprotective role in heart failure via A1 and A3 receptors by reducing cardiac hypertrophy, improving glucose homeostasis, reducing acidosis, improving calcium handling, improving mitochondrial function, reducing sympathetic stimulation and increasing natriuretic peptide release.116–119 However, increased circulating adenosine levels may have deleterious effects on the kidneys via A1 receptor-mediated vasoconstriction of the afferent arterioles, resulting in a decrease in glomerular filtration rate as well as sodium retention via the renin-angiotensin-aldosterone system.120 Therefore, raised adenosine levels may worsen volume overload and precipitate cardiorenal syndrome, although this may be ameliorated to some extent by A2 receptor-mediated vasodilatation.
Ageing
Population ageing is one of the most significant epidemiological pressures facing healthcare systems. During the century between 1900 and 2000, there was a 3.1-fold increase in the proportion of the population above 65 in the US.121 In Europe, where the population is the oldest worldwide, it is estimated that 25% of people will be over the age of 65 by 2030 – an approximate doubling.122 Ageing brings problems of an increased risk of pathology (especially ischaemic heart disease and heart failure), greater comorbidity, polypharmacy, impaired renal function, frailty and cognitive decline, as well as social complexities.
The prevalence of AF increases markedly from age 65 and 10% of individuals over the age of 80 have clinically overt AF; the number with unrecognised AF is likely greater.123 It has been suggested that AF itself contributes to the ageing process through to increased cerebral infarcts, tissue hypoperfusion and systemic inflammation.124 AF has been associated with dementia even in the absence of overt stroke.125 SCD also increases with age, with the annual risk seven times greater in 80-year-olds than in 40-year-olds.126 The elderly may gain less benefit from ICD therapy than younger patients.127
Aged myocardium is less tolerant to ischaemia and may show a blunted response to preconditioning.128 It has been suggested that adenosine is a key determinant of enhanced ischaemia tolerance. Aged hearts have greater interstitial levels of adenosine, potentially due to decreased cellular uptake, but produce less adenosine in response to adrenergic stimulation than young hearts.129 Age-related decline in cardioprotective adenosine response might be secondary to reduced downstream signalling from the A1 receptor and, in animal models, ageing lowers the anti-adrenergic effects of adenosine.130,131 However, adenosine remains safe and effective for the use of SVT management in the elderly.132
Adverse Effects and Contraindications of Adenosine
The short half-life of adenosine is advantageous when considering potential adverse effects. The side-effects of adenosine are unpleasant, but usually transient and tolerable. Chest pain, dyspnoea, cutaneous flushing and a sense of impending doom are well known. The most significant adverse effects are bronchospasm and arrhythmogenesis.
Bronchospasm and Pulmonary Effects
Caution has traditionally been exercised with adenosine regarding its propensity to cause bronchospasm in people with asthma or chronic obstructive pulmonary disease (COPD); recently, this practice has been questioned. Original reports suggested that inhaled adenosine, but not other nucleosides, could cause bronchoconstriction in patients with asthma but not in those with normal airway physiology. This appeared to have a maximal effect at 5 minutes after administration with partial recovery after 30 minutes.133 The mechanism proposed was a disturbance in the balance of the autonomic nervous system, which might favour bronchoconstriction, although other studies around that time had demonstrated increased sympathetic outflow without bronchoconstriction.74 A large, multicentre study of adenosine administration in patients undergoing nuclear imaging assessment of coronary perfusion reported that the incidence of bronchospasm was rare, occurring in only seven cases in more than 9,000 patients.134
Other later studies have somewhat refuted these claims. IV adenosine was shown to stimulate minute ventilation and the sensation of dyspnoea in patients with normal airways physiology, which is thought to arise from the activation of vagal C-fibres (J-receptors) and accounts for the well-documented sensation of dyspnoea.135 A study of IV adenosine in patients with asthma showed a similar but augmented response. Importantly, dyspnoeic sensations were not associated with objective spirometry measurements of airways obstruction.136 Animal studies in the rat, where small C-fibres of the vagus nerve were inhibited using capsaicin treatment, showed this eliminated adenosine-induced dyspnoea.137 Furthermore, blockade of the adenosine A1 receptor but not the A2 receptor prevented the response.138
The implication of such studies is that adenosine-induced dyspnoea, a C-fibre mediated response, might have been misinterpreted as bronchospasm in earlier reports, and therefore adenosine is likely to be safer in obstructive airways disease than previously supposed.
Nevertheless, case reports suggestive of bronchospasm continue to be published, mostly in patients with asthma or COPD.139,140 In the latter case, bronchoconstriction was sufficiently severe to cause respiratory failure requiring intubation; however, this was in the context of long-term theophylline use, suggesting that bronchoconstriction might have been a response to rapid theophylline antagonism. Another case report suggests an episode of profound bronchospasm in a young patient with apparently normal airways physiology, although the patient had a BMI of 40, which raises the possibility of obesity-related hypoventilation syndrome, obstructive sleep apnoea and obesity-related asthma.141 Additionally, no cross-sectional imaging or pulmonary function tests were presented to exclude underlying airways disease.
Furthermore, adenosine has recently been associated in the pathophysiology of severe COPD and idiopathic pulmonary fibrosis. In these conditions, CD73 (an enzyme involved in extracellular adenosine production) and adenosine A2 receptors are upregulated, with a particular preponderance in pulmonary macrophages. Adenosine is produced in response to cell damage, and upregulation of production and receptors allows for purinergic remodelling, which ultimately causes fibrosis.142 Similar findings have been reported in cystic fibrosis.143
It is rare that adenosine causes bronchoconstriction in patients with normal airways physiology. However, there are accounts of plausible harm caused in people with asthma and chronic lung disease, and those dependent on methylxanthines. Asthma is prevalent in children, where the most common arrhythmia is SVT. Adenosine should therefore be used with caution in these cases.1
Pro-arrhythmic Effects of Adenosine
Studies in emergency departments suggest adenosine induces arrhythmia in approximately 13% of those treated for SVT.42 These are mostly transient and benign, such as pauses, ventricular ectopy and non-sustained VT; however, major arrhythmias are also encountered, albeit rarely.144 Significant bradyarrhythmia in the form of sinus arrest, heart block and asystole have been reported.145–148
Adenosine-induced bradyarrhythmia may be more common when it is given concomitantly with other rate control medications and in patients with orthotopic liver transplantation, sepsis or heart failure.147,149–151 Adenosine administration has been associated with anoxic seizure in the context of bradyarrhythmia.152
Significant tachyarrhythmias have also been encountered in the form of torsades de pointes, sustained VT and VF.153–159 The risk of VF may be increased when adenosine is used in stable VT, pre-excitation or in patients with a prolonged QT interval.160–162 Several case series highlight the risk of inducing ventricular arrhythmia during fractional flow reserve measurements following intracoronary adenosine administration, suggesting that IV is a safer route or that lower doses are needed.163–166
Adenosine Following Cardiac Transplant
Concerns regarding adenosine in patients receiving a cardiac transplant have been raised because adenosine receptors are upregulated following parasympathetic denervation, potentially making adenosine more likely to cause asystole. This is of potential concern as up to 50% of cardiac transplant patients develop SVT, which reduces cardiac output and may result in invasive investigations for acute rejection. Alternatives such as CCAs may cause drug interactions with immunosuppressants and are contraindicated in infants. However, a recent case series of patients in sinus rhythm following cardiac transplant given adenosine showed no major adverse events, including asystole, or need for pacing, which calls these views into question.167
Transplant patients may require reduced doses, especially when the PR interval is prolonged. However, it should be noted that these studies were in young patients who did not have manifest SVT at the time of administration, so it remains to be shown if findings are translatable to the different neurohumoral circumstances present during SVT or in older populations.
Conclusion
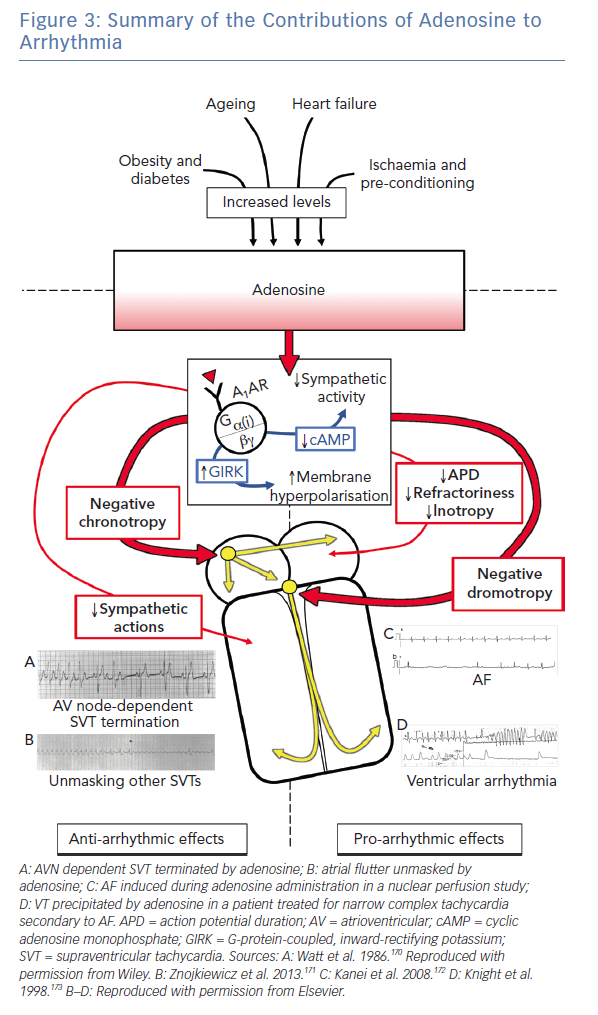
Adenosine celebrates its ninth decade of recognition this year. It continues to be a crucial member of the armamentarium in the management of arrhythmia (Figure 3), particularly SVT.1 Advances in metabolomics and receptor biology are now elucidating the critical role of adenosine in numerous common conditions related to arrhythmogenesis. In most cases, endogenous adenosine appears to play an anti-arrhythmic role, particularly in the context of ischaemia.168 Purinergic signalling pathways are likely to be future targets in the management of arrhythmia, both directly by actions on cardiac electrophysiology and indirectly in the treatment of its associated risk factors.
Clinical Perspective
- Adenosine is the first-line pharmacological therapy, following vagal manoeuvres where these are appropriate, in the treatment of acute atrioventricular node-dependent tachycardias, particularly atrioventricular re-entrant tachycardia and atrioventricular nodal re-entrant tachycardia, and has a termination rate of approximately 90%.
- Although administration is frequently associated with transient unpleasant symptoms, in the majority of cases adenosine is safe to use because of its short half-life. However, patients must be monitored for rare major adverse events, including arrhythmia, bronchospasm and severe dyspnoea.
- Calcium-channel antagonists are a good alternative to adenosine and have similar termination efficacy. They should be considered in patients with contraindications to adenosine or where a longer-acting pharmacological strategy is required.