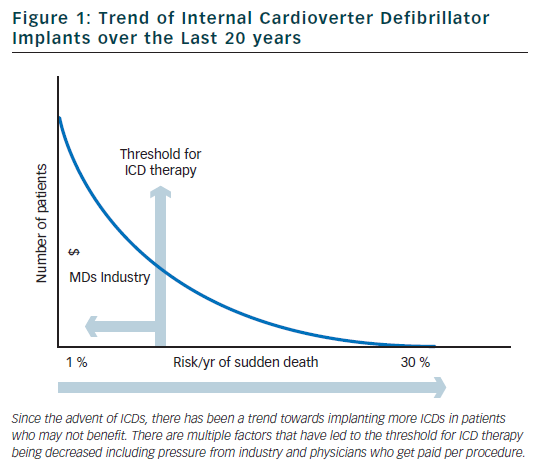
Sudden cardiac death (SCD) is one of the leading causes of death in developed countries. The yearly incidence of SCD is estimated to be 3,000,000 worldwide and between 300,000–450,000 in both the US and Western Europe.1,2 The survival rate for SCD is <1 % worldwide and close to 5 % in the developed world.3 In the past, cardiac arrest was thought to be most commonly due to ventricular fibrillation (VF) or ventricular tachycardia (VT) in approximately 75 % of cases.4–6 More recently, studies in the USA have suggested that the incidence of VF or VT as the presenting rhythm in out-of-hospital cardiac arrest has decreased to <30 % over the past thirty years.7–9 Globally, the incidence still probably exceeds 50 %.
Internal cardioverter defibrillators (ICDs) were invented in the early 1980s to be used to treat patients with ventricular arrhythmias refractory to medical therapy. Since that time, there has been a shift in philosophy away from medical and ablative therapies, and increasingly towards using ICDs for both primary and secondary prevention. Over the past 20 years, there has been a marked increase in the rate of ICD implants (figure 1).10 The increase in volume of ICD implants has mostly been from primary prevention ICDs which account for 80 % of all ICD implants in the US.11
In this review article, we will highlight the evidence for the use of primary prevention ICDs. We will also give a critical appraisal of the application of current ICD guidelines leading to abuse or misuse of ICD therapy.
Summary of Trials Evaluating Primary Prevention Internal Cardioverter Defibrillators
In selected populations, primary prevention ICDs have been shown to improve mortality. The multicentre automatic defibrillator implantation trial (MADIT) and multicentre unsustained tachycardia trial (MUSTT) included patients with infarct-related cardiomyopathy with reduced left ventricular systolic function (MADIT <35 % and MUSTT <40 %), documented non-sustained VT (NSVT), and inducible sustained VT during an electrophysiology study (EPS).12,13 In the MADIT trial, of the 196 patients with prior myocardial infarction (MI), asymptomatic NSVT and left ventricular ejection fraction (LVEF), <35 % were randomised to ICD therapy or conventional medical therapy if they had a positive EPS. The patients were followed for a mean of 27 months and the trial was ended early. This was due to a survival benefit noted in the ICD group compared to conventional medical therapy (15 deaths in the ICD group compared to 39 deaths in the conventional group).
The MUSTT study enrolled 704 patients with coronary artery disease (CAD), LVEF <40 %, asymptomatic NSVT and a positive EPS and randomly assigned them to antiarrhythmic therapy, antiarrhythmic therapy and ICD or no antiarrhythmic therapy. The patients who were randomised to ICD therapy had lower relative risk of arrhythmic events and of overall mortality (relative risk of 0.24 if ICD was implanted compared to those who did not receive a defibrillator). One limitation of both the MUSTT and MADIT studies is that the subjects did not receive what is now considered standard medical therapy. In particular, only 8 % of patients in the control group in the MADIT trial received beta blockers compared to 26 % of patients in the ICD group at the one month follow-up. Similarly, in the MUSTT trial, only 29 % of the EPS guided therapy group was prescribed beta blockers.
The coronary artery bypass graft (CABG) patch trial was another randomised trial that explored the possible benefits of primary prevention ICD in 900 patients who were undergoing CABG, had a LVEF <35 % and had an abnormal signal-averaged electrocardiogram (ECG). The CABG patch trial did not show any mortality benefit in primary prevention ICDs in the patient population being studied. It is possible that the LVEF and arrhythmogenic substrate improved after undergoing revascularisation with CABG.14 The mortality rate in the CABG patch trial was lower than in the MADIT study, likely reflecting the effects of revascularisation on mortality.
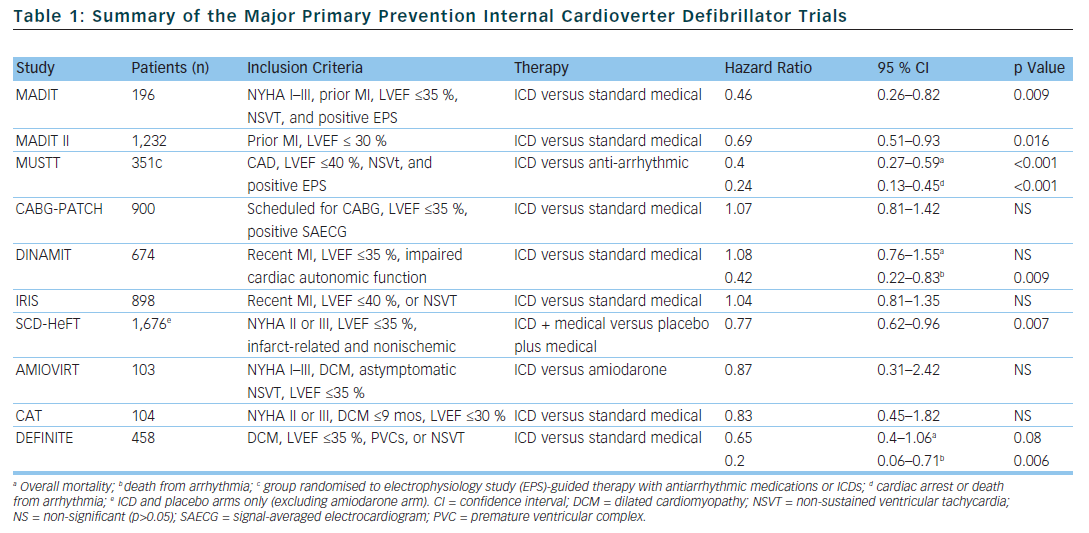
In the MADIT II trial, 1,232 patients with infarct-related cardiomyopathy and an EF <30 % were randomly assigned to either a primary prevention ICD or conventional medical therapy. At the 20 month follow-up, the ICD group had a 5.6 % lower mortality rate compared to the conventional medical therapy group.15 The results of the MADIT II trial have prompted clinicians to expand primary prevention ICD therapy to all patients with infarct-related cardiomyopathy and LVEF <30 %. However, 30 % of patients who received ICDs in the MADIT II study had NYHA class 3 or 4 heart failure and 18 % of the patients had left bundle branch block (LBBB) – a marker for LV dysfunction which is not caused by MI – making the results of the study difficult to expand to asymptomatic patients.
The risk of sudden cardiac death is highest within the 30 days following an acute myocardial infarction.16 Interestingly, a substudy of the MADIT II data showed that there was no benefit in ICD therapy less than 18 months after an acute MI.17 This, in part, may be related to the fact that at the time of enrollment, the average time post-MI was approximately 3 years. With this in mind, 2 trials were designed to evaluate the effectiveness of primary prevention ICDs in preventing SCD in the early post-MI period. The Defibrillator in acute myocardial infarction trial (DINAMIT) randomised 674 patients with recent MIs, a LVEF <35 % and impaired cardiac autonomic function (depressed heart rate (HR) variability or elevated average HR on 24 hour Holter) to ICD therapy or conventional medical therapy. During a mean follow-up period of 30 months, there was no mortality difference between the two groups. Although there were less arrhythmic deaths in the ICD group, there was an increase in non-arrhythmic deaths to offset any potential benefit in the ICD group.18
The results of the DINAMIT study were corroborated in the Immediate Risk Stratification Improves Survival (IRIS) study which was published in 2009. Patients were enrolled from 5–31 days after an acute MI if they had a LVEF <40 %, resting HR >90 bpm, or NSVT on 24 hour Holter. Prophylactic ICD therapy did not decrease the overall mortality. Similar to the DINAMIT study, the rates of SCD were lower in the ICD group, but the non-arrhythmic mortality rate was higher in the ICD group.19
Several trials have been designed to evaluate the efficacy of primary prevention ICDs in patients with non-ischaemic cardiomyopathy, however no randomised trial to date has shown a statistically significant advantage of primary prevention ICDs over medical therapy in preventing all-cause mortality. The Amiodarone Versus Implantable Cardioverter-Defibrillator: Randomized Trial (AMIOVIRT) and the cardiomyopathy trial (CAT) were both prematurely terminated due to lower-than-expected all-cause mortality rates. The AMIOVIRT trial enrolled 103 patients with NYHA I–III non-ischaemic dilated cardiomyopathy, LVEF≤ 35 %, and asymptomatic NSVT to ICD or amiodarone. There was no benefit with ICD therapy (Hazard ratio of 0.87 with 95 % CI of 0.31 to 2.42).20 The CAT trial randomised 104 patients with NYHA class II–III dilated cardiomyopathy <9 months, and LVEF ≤30 % to ICD or standard medical therapy. Once again ICD therapy did not confer a mortality benefit (HR of 0.83 with 95 % CI of 0.45–1.82).21
The Defibrillators in non-ischaemic cardiomyopathy treatment evaluation (DEFINITE) study randomised 458 patients with dilated cardiomyopathy with a LVEF ≤35 % and PVCs or NSVT to ICD or standard medical therapy. There was a significant benefit in preventing arrhythmic deaths, but a statistically insignificant effect in decreasing all cause mortality.22 The Sudden cardiac death in heart failure trial (SCD-HeFT) is the largest study that enrolled patients with non-ischaemic dilated cardiomyopathy. SCD-HeFT randomised patients with NYHA II–III non-ischaemic or infarct-related cardiomyopathy with LVEF ≤35 % to ICD or standard medical therapy. There was a 23 % relative risk reduction in death in the ICD group compared to placebo. Of the patients that enrolled in the SCD-HeFT study, 48 % had non-ischaemic cardiomyopathy. The non-ischaemic cardiomyopathy subgroup had a non-significant reduction of overall mortality (Hr 0.73 with 95 % CI of 0.5–1.07).23
In summary, although there is a trend toward improved survival in patients with non-ischaemic cardiomyopathy who have been treated with primary prevention ICDs, there has never been a trial showing a statistically significant overall mortality benefit (not including cardiac resynchronisation therapy trials). The only patients that have been shown to derive statistically significant survival benefit from primary prevention ICDs in the modern era (reperfusion and routine beta blocker therapy) are high risk patients with infarct-related cardiomyopathy and LVEF ≤35 %.
Quality care or Misuse/Abuse?
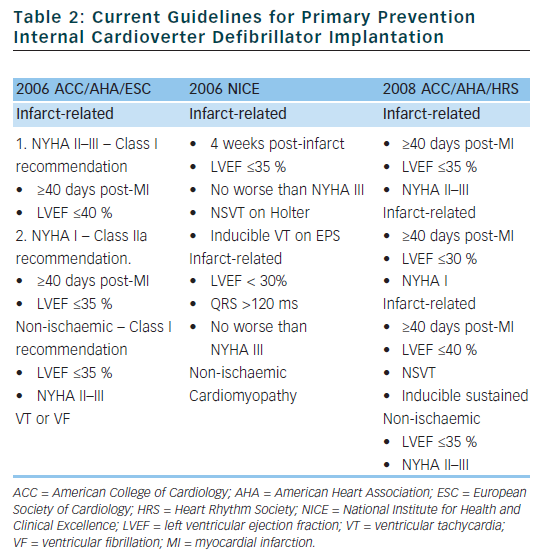
Based on the clinical trials described above, guidelines have been established to assist physicians in determining which patients may benefit from primary prevention ICD implantation (see Table 2).24,25 Although the rates of SCD are similar in the US and Western Europe, the rate of ICD implantation in the US was more than five times that of Western Europe in 2006 (577 per million compared to 155 per million).26 There are many factors that contribute to the disparity in ICD implant rates in different regions. Some of the factors that are likely contributing to the disparate use of ICDs include: variation in guidelines, fear of litigation, differences in compensation models (paid per implant in many areas of the US), and pressures from local culture and device manufacturers. Although the current guidelines were established to help choose appropriate candidates for ICD implantation, there are several limitations which we will discuss in detail in the following sections.
Limitation of Ejection Fraction as a Sole Marker of Sudden Cardiac Death
There are multiple risk factors that contribute to a patient’s overall risk for developing SCD. However, the guidelines for primary prevention ICD implant rely heavily upon a patient’s EF as the main risk factor to consider. A recent retrospective analysis of the MUSTT database showed that patients with an EF, <30 % and no other risk factors, would have a 2 year arrhythmic death risk of less than 5 %.27 Many patients who are “eligible” for primary prevention ICD implant based on current guidelines were either under-represented or showed no benefit in randomised controlled trials. Although trials evaluating the efficacy of primary prevention ICDs enrolled patients with EFs up to 40 %, the majority of patients actually enrolled in the trials had EFs substantially lower than the upper limits allowed to be enrolled in the studies.28
Elderly Patients are Under-studied
The major clinical trials evaluating the efficacy of primary prevention ICDs did not exclude patients based on age alone, however the mean age of patients in these trials was <65 years. In contrast to the patient populations that were studied in the ICD trials, a recent review of the National Cardiovascular Data Registry (NCDR) and Advancements in ICD Therapy Registry (ACT) (US patients) showed that >40 % of ICD and Cardiac Resynchronisation Therapy Defibrillator (CRT-D) devices are implanted in patients over the age of 70 years and >10 % in patients over the age of 80 years.11
Early data suggested that elderly patients are much more likely to die from other causes besides SCD and are less likely to benefit from ICD therapy compared to younger patients.29 More recent studies evaluating the efficacy of ICD therapy in the elderly have confirmed that elderly patients are less likely to derive any total mortality benefit from an ICD and are more likely to suffer a non–arrhythmic death.30,31 Although elderly patients may receive appropriate therapies from ICDs, it appears that ICDs do not effect overall mortality rates, but rather change the mode of death.32
Co-morbidities Influence Whether a Patient Will Benefit from Primary Prevention Internal Cardioverter Defibrillator Implant
There are multiple characteristics that influence whether a patient would potentially benefit from a primary prevention ICD. A risk stratification score was developed using the MADIT II trial data in 2008 that used multiple variables to predict benefit of primary prevention ICD implant.33 The risk score model included five variables (NYHA >II, age >70 years, blood urea nitrogen [BUN] >26, QRS >120 ms and atrial fibrillation [AF]). The investigators found a U-shaped pattern of ICD efficacy with the lowest risk (0 variables) and highest risk patients (BUN >50 and/or creatinine 2.5 mg/dl) not benefiting from primary prevention ICD.
Another group of patients that is either not represented or under-represented in primary prevention ICD trials are those with advanced chronic kidney disease (CKD) or with end-stage renal disease on hemodialysis.34 Patients with CKD are at much higher risk than the general population to develop heart failure and also to suffer SCD (6 to 9 fold increase).35 The only data addressing kidney disease from the major randomised controlled trials comes from a subgroup analysis of the MADIT II study.36 The retrospective analysis showed that patients with a glomerular filtration rate (GFR) >35 ml/min/1.73 m2 benefited from ICD therapy (overall risk reduction for all-cause mortality 32 % p=0.01 and overall risk reduction for SCD of 66 % p <0.001). However patients with a GFR <35 ml/min/1.73 m2 did not benefit (all-cause mortality hazard ratio 1.09, p=0.84; SCD hazard ratio 0.95, p=0.95). Aside from a life expectancy of >1 year, current guidelines do not address patient co-morbidities when deciding whether or not to implant a primary prevention ICD.
Acute Complications at the Time of Internal Cardioverter Defibrillator Implant
When considering the possible benefits of primary prevention ICDs, it is also critical to weigh the risks of ICDs. Acute, implant-related complications include pocket hematoma, subclavian thrombosis or pulmonary embolism, central venous injury leading to hemothorax, cardiac perforation, lead dislodgment, paradoxical embolism or complications related to defibrillator threshold (DFT) testing (i.e. post-shock pulseless electrical activity (PEA), refractory VF, or embolism from conversion of AF), and rarely death.37 Acute complications occurred in approximately 5 % of patients in the SCD–HeFT trial and 2.5 % of the MADIT II patients.15,23
Infection
ICD infection is an uncommon but serious complication that is associated with a high mortality rate.38 Patients with diabetes and renal disease are at increased risk for infection. Additionally elderly, frail patients are at higher risk of pocket erosion (particularly women). Studies have shown that the rate of device infections is increasing over time.39,40 The rate of device infections was close to 1 % in a recent study evaluating device implants at a large tertiary care centre in Canada. Infections were more likely to occur after a generator change or implant of a dual chamber or CRT device.41 The standard treatment for device related infections often involves complete system extraction which can be a costly and dangerous procedure with major complication rates up to 2 % and mortality rates up to 0.8 % as reported in the two major clinical trials.42–43
Inappropriate Shocks and Proarrhythmia
In both the MADIT II and SCD-HeFT trials, the rates of inappropriate ICD therapy were remarkably high with close to 15 % of all patients receiving an inappropriate ICD shock during the study period.44–45 For unclear reasons, the mortality rate is increased in patients who receive inappropriate ICD therapy nearly two-fold. This finding has been corroborated in other clinical trials.46 Inappropriate ICD shocks can lead to further arrhythmias (if the shock is not timed appropriately). Additionally, the ICD lead and pacing have also been implicated as possible causes of arrhythmia.47,48 The psychological effects of ICD therapy can be disabling. Up to 40 % of patients who receive ICD therapies go on to develop anxiety, depression, or post-traumatic stress disorder.49
Generator and Lead Failures/Recalls
Recently, there has been a lot of attention given to lead and generator recalls by the device manufacturers, especially with the recall of the Medtronic Fidelis and St Jude Riata leads.50,51 Estimates of ICD lead failure have been estimated to be as high as 20 % at 10 years.52 Additionally, up to 40 % of leads or generators implanted in patients are affected by FDA safety advisories.53 Most patients can be managed expectantly who have implanted devices or leads that are under recall. However, complications related to lead or device failure can result in death. Hauser et al. reported 22 deaths related to failure of Riata leads leading to short circuiting between high voltage components.54 Device and lead failure often require lead extraction. System explant and lead extraction related to recalls and lead failures is associated with significant cost, morbidity, and mortality.55
Cost-effectiveness
There have been large discrepancies in cost-effectiveness models based on the study population being analysed. A Markov model constructed from eight randomised trials was developed by Sanders et al. in 2005 that showed a cost-effectiveness ratio below $100,000 as long as the ICD reduced mortality for at least 7 years.56 In general, cost effectiveness ratios are considered acceptable in the US as long as the ratios are less than $100,000. Another cost effectiveness model was constructed using the data from MADIT II and it found that the cost-effectiveness ratio was $235,000 over the 3.5 year duration of the trial. If the time interval was stretched out to 12 years, then the cost-effectiveness ratio varied between $78,600 and $114,000.57
European cost-effectiveness models have also been established. A model using Belgian national references has been established that estimated mean lifetime cost per QALY gained as €31,717.58 A more recent model was developed using the 8 year follow-up data from MADIT–II applied to Germany. The estimated cost-effectiveness ratio was €44,736 which was considered too expensive to recommend broad implementation (the cut-off in Europe is typically €40,000).59 The key to keeping cost effectiveness ratios down is to implant devices in moderate risk patients who would be more likely to use the ICD, but not likely to die in the near future.
Conclusions
Although primary prevention ICDs have been shown to benefit selected populations, the current guidelines and medical culture have expanded ICD use to patient populations that have not been adequately studied and may not benefit from ICD implantation (misuse or abuse). The current guidelines rely heavily upon LVEF which by itself does not adequately predict which patients may benefit from primary prevention ICDs. The ICD guidelines also do not address important issues such as age and co-morbidities. The magnitude of mortality benefit has been over-exaggerated and the morbidity from complications and ICD system failures has been under-appreciated. Cost-benefit analyses have also assumed best-case scenarios and likely do not reflect real-world practice.
Primary prevention ICDs may modestly prolong life, however this needs to be weighed against the potential morbidity and diminished quality-of-life from procedures and ICD shocks. It is important to understand that patients who do not experience benefit from ICD therapy are still exposed to risks of the device. An honest dialogue between physician and patient addressing these issues should occur prior to implantation of ICD.
Clinical trial data has quickly been implemented into guidelines without critical reassessment of the strengths and limitations of the evidence. ICD therapy has inherent risks including infection, unnecessary shocks, potential for proarrhythmia, device malfunction, highly publicised manufacturer advisories, and procedural complications, which can adversely affect morbidity, mortality, and quality-of-life.
A re-appraisal of the benefits and potential hazards of ICD therapy is necessary to enable physicians to a have a more mutually informed and balanced dialogue with their patients.