Cardiomyopathies are heterogeneous heart muscle disorders with a wide range of aetiologies and clinical manifestations. They are often defined by their causes (i.e. hypertension, prior myocardial infarction, valvular heart disease), although current major society definitions describe cardiomyopathy as the presence of abnormal myocardial structure and/or function in the absence of underlying structural heart disease. Long-standing tachycardia is a well-recognised cause of heart failure and left ventricular dysfunction, and has led to the nomenclature, tachycardia-induced cardiomyopathy (TIC). TIC is generally a reversible cardiomyopathy if the tachycardia can be treated effectively, either with medications, surgery or catheter ablation. TIC can also manifest in patients with baseline left ventricular dysfunction from underlying structural heart disease that develop a worsening of their myocardial dysfunction in the setting of prolonged tachycardia, which can be reversed with control of the tachycardia. The diagnosis is usually made after demonstrating recovery of left ventricular function with normalisation of heart rate in the absence of other identifiable aetiologies.
TIC was first described in 1913 in a young patient who presented with congestive heart failure (CHF) and atrial fibrillation (AF) with rapid ventricular response.1 In the subsequent decades, further reports were written documenting cases of complete resolution of CHF after cardioversion of AF to sinus rhythm.2–4 A century after the first reported case, we are still working to expand our understanding of the mechanisms underlying this disorder. A variety of chronic or incessant tachyarrhythmias have been implicated in the pathogenesis of TIC, the most classic being AF,5–7 atrial flutter,8 incessant supraventricular tachycardia,9–12 ventricular tachycardia13–16 and premature ventricular depolarizations17 (see Table 1). The most common presentation is a dilated cardiomyopathy with or without the causative tachycardia. Given that this diagnosis represents a potentially reversible cause of heart failure, its recognition is critically important. In this review, we will discuss the proposed mechanisms of TIC, the causative tachycardias and their treatment, outcomes for patients diagnosed with TIC, and future directions for research and clinical care.
Experimental Models
Background
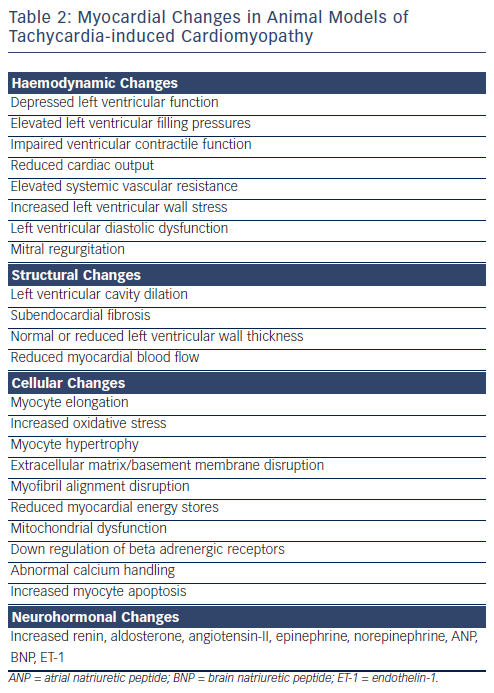
The first experimental model of TIC was described by Whipple et al. in 1962 where he demonstrated that rapid, prolonged atrial pacing resulted in low output heart failure.18 This model has since become widely used as a model to study CHF. Animal studies, primarily in dogs and pigs, have shown that sustained rapid atrial or ventricular pacing results in systolic heart failure that is neurohormonally and haemodynamically similar to left ventricular systolic dysfunction in humans.19–23 The majority of what is known about the pathophysiologic mechanisms underlying TIC is supported by pacing-induced heart failure in animal models (see Table 2). It is important to recognise that although rapid pacing likely provides a close approximation to the effects of native tachyarrhythmias, this supposition cannot be definitively confirmed. Asynchronous ventricular pacing as well as atrioventricular (AV) sequential pacing at physiologic heart rates have been shown to be associated with increased rates of heart failure.24
The mechanism of heart failure development in chronic right ventricular pacing is not entirely known but may in part be due to an abnormal activation pattern of the ventricle. This hypothesis is supported by the fact that various forms of abnormal ventricular activation have been shown to adversely affect left ventricular systolic function including chronic right ventricular pacing,25–27 left bundle branch block28,29 and ventricular pre-excitation.30,31 Given this, chronic atrial pacing is likely a more pure model of TIC, as it should result in fewer electromechanical changes in the ventricles beyond increased rate.
Haemodynamic Changes
Sustained rapid atrial or ventricular pacing can produce severe biventricular systolic and diastolic dysfunction in animal models. The degree of ventricular dysfunction as well as its onset appear to be related to the rate and duration of pacing. By pacing at a slower rate or for a shorter duration, a lesser degree of left ventricular dysfunction can be produced.32–35 Although both chronic atrial and ventricular pacing result in left ventricular dysfunction, deterioration of left ventricular ejection fraction (LVEF) and left ventricular cavity dilation are more marked with chronic ventricular pacing than chronic atrial pacing.36 Intermittent ventricular pacing appears to produce a less advanced syndrome of heart failure compared with continuous ventricular pacing.37 As noted above, the differential effects between chronic atrial and ventricular pacing may be due to the effects of chronic dyssynchronous left ventricular contraction with ventricular pacing.
The haemodynamic consequences of prolonged pacing in animal models include markedly elevated left ventricular filling pressures,38–40 impaired ventricular contractile function,23,40–43 reduced cardiac output, elevated systemic vascular resistance,20,21,38,42 and increased left ventricular wall stress.34,42,44,45 Loss of myocardial contractility has also been demonstrated as shown by a diminished or absent response to inotropic agents, volume loading and postextrasystolic potentiation.46 Disturbed elastic properties of the left ventricle (LV) have also been reported, which result in a stiffer ventricle and worsening LV diastolic function. In a normal heart, LV torsion reduces transmural fiber strain during systole, and recoil in early diastole is thought to enhance left ventricular filling. Animal models of TIC have shown that LV torsion decreases in the setting of pacing-induced heart failure, and with it the degree of diastolic recoil.47 These changes have been attributed to changes in the geometric orientation of the ventricle with changes in cardiac structural proteins as well as alterations in basement membrane and extracellular matrix function.48,49 As a response to these changes, similar to other forms of CHF, pacing-induced CHF in animal models results in upregulation of the neurohormonal axis leading to elevated levels of serum atrial natriuretic peptide (ANP), renin, aldosterone, angiotensin-II, epinephrine and norepinephrine.50,51 Interestingly, the vasodilator, natriuretic and renin-lowering effects of ANP are blunted in the canine model of TIC due to reduced cyclic guanosine monophosphate (GMP) expression in response to ANP.52Atrial tissue levels of ANP are reduced while serum levels of ANP are elevated. Brain natriuretic peptide (BNP) levels are also elevated, though to a lesser extent than ANP.53
Structural Changes
The most pronounced structural change in TIC is left ventricular cavity dilatation.33,38–40,44 LV dilation is more marked for end-systolic than end-diastolic volumes,41,42 and produces a spherical chamber geometry.38,39,54 Commonly, left ventricular cavity dilation is accompanied by little or no change in LV wall mass with a normal or reduced LV wall thickness.22,39 Cellular changes accompanying the change in chamber size include cellular elongation, decreased myocyte cross-sectional area and disruption of normal myofibril alignment.23,46 Other studies have reported increased numbers of cells in both the longitudinal and transverse sections of intact myocardium from animals with pacing-induced heart failure.55 Chamber dilation and wall thinning has been shown to correlate with loss of myocytes and an increase in volume of the remaining myocytes,56 although other studies have shown remodeling without myocyte hypertrophy.57 Some of these changes may be due to changes in the extracellular matrix with reduction in myocyte attachment to the basement membrane. Mitral regurgitation has also been seen, likely as a result of an increase in left ventricular dimensions.38 This may then contribute to further left ventricular dilation and dysfunction.
Myocardial ischaemia has also been proposed as a mechanism for TIC. Changes in the structure, distribution and function of the coronary vasculature in TIC have been demonstrated, including abnormal subendocardial and subepicardial blood flow ratios, and impaired coronary flow reserve.58,59 These changes may impair myocardial blood flow and limit oxygen delivery, accelerating myocardial injury and worsening ventricular dysfunction. However, it is not clear whether these changes truly contribute to myocardial dysfunction or are a result of increased myocardial demand with rapid pacing and decreased myocardial supply due to elevated ventricular filling pressures and decreased cardiac output.
Increased areas of fibrosis have been seen along the subendocardial region of animal hearts with pacing-induced heart failure.23 It may be that increased levels of angiotensin II and aldosterone in this setting trigger increased myocardial fibrosis, as is the case in heart failure of various aetiologies. A significant increase in the number of ventricular myocytes undergoing apoptosis has been shown to be present in TIC.60
Cellular Changes
Reduced myocardial energy stores have been implicated in the pathogenesis of TIC including decreased levels of creatine, phosphocreatine, adenosine triphosphate and glycogen, as well as enhanced activity of Krebs cycle oxidative enzymes and decreased activity of the sodium-potassium adenosine triphosphatase (Na-KATPase)pump. These changes are likely related to alterations in cellular metabolism in the setting of mitochondrial injury and decreased mitochondrial activity with increased activity of the Krebs cycle and oxidative stress.19,23,43,61 Increased levels of oxidative stress have been shown to accompany higher degrees of myocyte apoptosis in animal models of TIC. Chronic myocardial stimulation at high rates is believed to cause mitochondrial DNA oxidative damage preferentially given its higher sensitivity compared with nuclear DNA.62 Treatment with selegiline, an inhibitor of monoamine oxidase, has been shown to decrease oxidative stress, myocyte injury and apoptosis.63 Similar results have been shown with the administration of antioxidant vitamins to animals with TIC leading to reduced levels of oxidative stress and attenuated cardiac dysfunction. These effects were not observed in sham-operated animals.64 Elevated endothelin-1 (ET-1) levels have also been reported in animal models of pacing-induced heart failure, and seem to hasten the progression of TIC. ET-1 may play a role in mitochondrial dysfunction as it has been shown to produce mitochondrial changes in the electron transport chain.65 Downregulation of beta-adrenergic receptors and a resultant decreased sympathetic responsiveness has also been described in animals with TIC.66,67 This reduction in beta receptor density and responsiveness to beta-adrenergic stimulation has been shown to be independent of haemodynamic and neurohormonal factors.68 It has also been shown to normalise in the setting of rate control.69 However, it is not clear whether these changes contribute to myocardial dysfunction or whether they are a consequence of prolonged rapid pacing and chronically enhanced sympathetic activity.70
Abnormal calcium handling also seems to play a role in the pathogenesis of TIC. Extensive abnormalities in calcium channel activity and calcium transport in the sarcoplasmic reticulum have been seen as early as 24-hours after the initiation of rapid pacing. It appears that the severity of calcium cycling abnormalities correlates with the degree of ventricular dysfunction.43,71 Downregulation of calcium cycling has been correlated with lower activity of sarcoplasmic reticulum calcium transport adenosinetriphosphatase (ATPase) and myofibrillar calcium ATPase.72 Decreased availability of calcium to myocytes may lead to the reduction in contractility seen as a result of TIC.70 Isolated ventricular myocyte preparations have demonstrated decreased density of T-tubules and L-type calcium channels resulting in abnormal excitation–contraction coupling.73 It is important to recognise that many of the changes reported above are not unique to TIC, but are seen in multiple forms of chronic heart failure and may be related, at least in part, to the downstream effects of elevated filling pressures and decreased cardiac output rather than the tachycardia itself. Changes seen early after initiation of rapid pacing are more likely to be related to elevated heart rates themselves, whereas later changes are more likely to be due to a combination of elevated rates as well as the downstream effects of the heart failure syndrome.
Arrhythmogenesis
The heart failure state leads to an arrhythmogenic substrate due to the electrical heterogeneity in the myopathic ventricle. Abnormalities in repolarization have been implicated in the genesis of ventricular arrhythmias in all forms of heart failure, with prolonged repolarization being the most frequently reported. Repolarization abnormalities have been demonstrated in a porcine model of TIC evidenced by prolonged QTc intervals, reduced transmural gradients and decreased spatial dispersion of repolarization. Despite these changes, in this study, no deaths due to polymorphic ventricular tachycardia (VT) were observed and the authors concluded that repolarization was uniformly prolonged in their TIC model, attenuating the animals’ predisposition to bradycardia-dependent arrhythmias.74 However, conflicting findings were found in a canine model of TIC where heterogeneous repolarization abnormalities were seen with subsequent development of polymorphic VT. As is the case with all forms of heart failure, in this study, the incidence of VT was increased as heart failure progressed.75 The question remains as to whether TIC has a different risk of ventricular arrhythmias and sudden death compared with other forms of heart failure. Rates of malignant arrhythmias and sudden death are likely to be related to the severity of myocardial dysfunction and heart failure, and therefore may substantially decrease with resolution of TIC.
Time Course and Recovery
As noted above, the haemodynamic consequences seen in animal models of TIC can be seen as early as 24 hours after the initiation of rapid pacing. Some haemodynamic changes, such as increased intracardiac filling pressures, increased pulmonary artery pressures and decreased systemic arterial pressures generally plateau at one week, whereas cardiac output, ejection fraction and cardiac volumes may continue to worsen for up to 3–5 weeks.33,38,40,50 Changes in intracardiac filling pressures, cardiac output and systemic vascular resistance are generally reversible with cessation of rapid pacing, although in some cases ejection fraction may not return to baseline and abnormalities in contractile function may persist.33,76 Within 48 hours of cessation of pacing, intracardiac filling pressures, systemic arterial pressures, systemic vascular resistance and cardiac index have been shown to return to levels similar to control animals.34
Significant improvements in LVEF have been shown by 24–48 hours with normalisation after 1–2 weeks.34,41 However, residual contractile dysfunction has been seen to persist in isolated myocytes for up to four weeks.77 Within four weeks, all haemodynamic variables return to control levels while end-systolic and end-diastolic volumes remain elevated twelve weeks after termination of pacing.33,41,42,54 Left ventricular hypertrophy has been shown to develop after the cessation of rapid pacing. The mechanism of this hypertrophy seen during recovery is not known but may be due to an inability to respond to hypertrophy signals during pacing or potentially a compensatory remodeling response.32,54,77 Proliferation of large collagen fiber bundles after termination of pacing has been seen and may contribute to the left ventricular hypertrophy noted during recovery.78
Premature Ventricular Contractions and Tachycardia-induced Cardiomyopathy
Less is known about the effects of premature ventricular contractions(PVCs) on LV function given the smaller number of animal models in this setting. A recent study published the results of a canine model of PVC-induced cardiomyopathy through the use of right ventricular (RV) apical pacing in a bigeminal pattern. All animals with PVCs developed reduced LVEF and enlarged LV systolic dimension. In all dogs with PVCs, the cardiomyopathy was reversible four weeks after cessation of PVCs. Despite the development of LV dysfunction based on echocardiography, the dogs with PVC-induced cardiomyopathy did not show increased inflammation, fibrosis or apoptosis compared with control animals. Inflammatory infiltrates were absent and there was no evidence of abnormal mitochondrial phosphorylation. They did find that ventricular refractory periods prolonged in the PVC-induced cardiomyopathy model suggesting some amount of electrical remodeling, potentially related to alterations in intracellular ion channels. The authors concluded that the abnormalities in PVC-induced cardiomyopathy are functional ratherthan structural based on these findings.79 However, further research to replicate and expand on these findings is necessary.
Human Studies
Multiple arrhythmias have been associated with TIC including AF, incessant supraventricular tachycardia of various forms and VT. Additionally, premature ventricular beats of sufficient frequency have also been associated with the development of TIC in humans. Restoration of sinus rhythm, control of the ventricular response or decrease in the frequency of premature contractions all result in an improvement in left ventricular function and clinical heart failure. Despite reports of reversible cardiomyopathy in the setting of chronic tachycardia for the last hundred years, prospective studies of TIC did not begin until the last few decades, and many reports are descriptive in nature with interpretation and application limited by small study size. TIC can occur at any age and has been reported as early as in utero80 but is also seen in infants, children81–83 and adults. The incidence of TIC is unknown, given that most reports in the literature are small retrospective series or case studies involving individual patients. Estimations of incidence are also limited by the fact that TIC is a diagnosis of exclusion and no single test can be performed to confirm or refute its presence. TIC is likely an under diagnosed phenomenon with the true incidence being higher than what has been reported thus far in the literature.
Atrial Fibrillation
The most rigorously studied aetiology of TIC in human subjects is chronic AF. AF is known to increase risk of heart failure irrespective of the heart failure aetiology.84 However, in a certain subset of patients with heart failure and AF, restoration of sinus rhythm or control of ventricular rates will markedly improve or normalise left ventricular function suggesting the tachycardia itself as the underlying cause of the myopathy. Early studies of cardioversion for AF demonstrated an improvement in cardiac output, cardiopulmonary exercise testing and LVEF with return to sinus rhythm.85–88 A proportion of patients with ‘idiopathic’ dilated cardiomyopathy and chronic AF will have a significant increase in LVEF after pharmacologic or electrical conversion to sinus rhythm. A study assessing the time course of improvement in left ventricular function following cardioversion of AF to sinus rhythm demonstrated that atrial systolic function improved one week after return to sinus rhythm, whereas LVEF and peak oxygen consumption lagged behind and did not show improvement until one month following cardioversion. These results suggested the presence of an underlying ventricular myopathy causing heart failure rather than only the loss of atrial contractile function and atrioventricular synchrony.89 Further support of this concept has been shown in studies of chronic AF and heart failure treated with AV junction ablation and permanent ventricular pacing where a similar improvement in left ventricular function is seen with normalisation of ventricular rates irrespective of the atrial rhythm and function.6,90–93
However, it seems that rate alone is not the only causative factor in TIC. In a small series of patients with AF and a controlled ventricular response, AV junction ablation and pacemaker implantation resulted in a significant improvement in LVEF, fractional shortening and functional capacity suggesting that regularity and not rate alone has an effect on left ventricular function in chronic AF.94 A more recent study of catheter ablation for AF reported an improvement in LV function following catheter ablation. This study also found that only a minority of the patients with depressed LV function had elevated ventricular rates on routine monitoring prior to ablation.95 Another important insight derived from this study is that improvement in LV function following return to sinus rhythm was not an artifactual change related to difficulty in interpreting LV systolic function by echocardiogram when in AF, as this study measured LV function in sinus rhythm one day following the ablation as well as six months later. Although in this instance, one could also question whether the ejection fraction post-procedure was an adequate representation of pre-procedure LV function.96
Atrial Tachycardia
Incessant atrial tachycardia (AT), although a relatively uncommon supraventricular tachycardia, is a well-known cause of TIC. The term incessant refers to an AT that is present at least 90 % of the time.97 The arrhythmogenic mechanism is generally increased automaticity of an ectopic atrial pacemaker.98 Rates tend to be related to levels of alertness and activity, and may increase during pregnancy as the ectopic focus tends to be sensitive to autonomic modulation. Myocardial dysfunction in the setting of incessant AT has been reported in approximately 10 % of patients and has been shown to be more prevalent in younger patients.99 Surgical treatment of incessant AT has been reported to result in improvement in dilated cardiomyopathy.11,100,101 Advancements in catheter ablation have allowed for definitive therapy of incessant AT without requiring surgery, which has also been shown to result in normalisation in LV function in a majority of patients.9,10 The most recent series of incessant AT patients reported normalisation of LV function in 97 % of patients after successful ablation.99
Reentrant Supraventricular Tachycardias
Reentrant supraventricular tachycardias, including atrioventricular nodal reentrant tachycardia (AVNRT) and atrioventricular reciprocating tachycardia (AVRT) are most commonly paroxysmal but rarely can be incessant in nature. The latter category would include persistent junctional reciprocating tachycardia (PJRT), a form of incessant AVRT. When cardiomyopathy develops in this setting, the arrhythmias are generally incessant. Given that incessant reentrant supraventricular tachycardias are less common, they are also less well studied, with smaller series of patients reported, but TIC has been reported in the setting of AVNRT, AVRT and PJRT.102–106 As is the case with TIC and incessant AT, definitive treatment of the causative arrhythmia with pharmacologic suppression,105 surgery102,103 or catheter ablation,103,104,106 results in reversal of left ventricular dysfunction (see Figure 1). In general, once these arrhythmias become incessant, pharmacologic suppression is difficult and definitive treatment with catheter ablation is recommended.
1:2 Non-reentrant Dual Atrioventricular Nodal Tachycardia
Dual AV nodal physiology provides the substrate for the most common paroxysmal supraventricular tachycardia, AVNRT. As noted above, it is rare for AVNRT to become incessant and lead to TIC. However, dual AV nodal physiology can also facilitate the development of TIC through an incessant form of non-reentrant AV nodal tachycardia. In this scenario, a single sinus beat may result in two ventricular depolarizations with double antegrade conduction via fast and slow pathways.107 1:2 tachycardia occurs when this phenomenon repeats with such frequency that tachycardia ensues (see Figure 2). The exact prevalence of 1:2 non-reentrant dual AV nodal tachycardia is difficult to determine as published studies of this tachycardia are limited, making the frequency of TIC in this setting difficult to determine. In a recent review of the literature, 44 cases of 1:2 nonreentrant dual AV nodal tachycardia were described between 1970 and 2010. Of these 44 cases, eight patients had a reduced LVEF <45 %. All eight of these patients underwent catheter ablation of the slow AV nodal pathway with subsequent normalisation of left ventricular function in all cases (see Figure 3). A ninth patient was reported to have normalisation of left ventricular dysfunction after rate control alone.108
Ventricular Tachycardia
Reports of TIC in the setting of sustained monomorphic VT are much less common than with supraventricular tachycardias, since sustained VT is most often associated with some form of underlying structural heart disease. When VT leads to TIC, it is generally idiopathic and most commonly originates from the right ventricular outflow tract, left ventricular outflow tract or coronary cusps. Rarely, these arrhythmias may become persistent or repetitive enough to result in reversible left ventricular dysfunction.13,15,16 A recent single-centre series of 249 patients without overt structural disease and frequent monomorphic PVCs or repetitive sustained VT reported sustained monomorphic VT with or without PVCs in 11 % of patients. Only 7 % of patients had TIC. However, the presence of repetitive monomorphic VT was a significant predictor of TIC, particularly when it was the predominant arrhythmia on 24-hour Holter monitoring.109 As is the case with supraventricular arrhythmias, left ventricular dysfunction generally normalises following ablation of the arrhythmia.15,16,110
Premature Ventricular Contractions
PVCs have been associated with the development of TIC in the absence of sustained ventricular arrhythmias, and the severity of TIC is generally related to the burden of ventricular ectopy.109,111–113 Although the number of ventricular activations may increase with frequent PVCs, true tachycardia may not be present despite development of cardiomyopathy suggesting that, as is the case with AF, other factors must play a role in the development of LV dysfunction beyond rate. It has been postulated that electrical activation originating within the ventricular myocardium due to PVCs causes an inefficient mechanical ventricular contraction lacking synchronous myocardial activation. When persisting on a repetitive long-term basis, dyssynchronous ventricular contraction presumably leads to deterioration in LV function through remodeling effects. This is supported by the fact that similar adverse effects of abnormal ventricular activation on left ventricular systolic function have been observed in patients with chronic right ventricular pacing,25–27 left bundle branch block28,29 and ventricular pre-excitation30,31 as noted previously.
As with idiopathic VT, idiopathic PVCs have a predilection for the outflow tracts and coronary cusps, although the development of TIC is thought to be irrespective of the PVC origin.114,115 Several studies have shown that PVC frequency correlates with extent of LV dysfunction. Patients with decreased LVEF at the time of presentation have been found to have a higher mean PVC burden as compared with those with normal LV function.111,113,116 However, a clearly defined cut-off at which time TIC is likely to develop has not been determined nor has the approach to this definition. Some studies have focused on PVC burden as a percentage of total ventricular activations while others have examined the absolute number of PVCs in a 24-hour period. An early study reported that patients with a PVC burden of 20 % were more likely to have impaired LV function compared with those patients with a PVC burden of <20 %.76 A later study found that a PVC burden of 16 % by a receiver operating characteristic (ROC) curve analysis best separated patients with and without TIC.109 Most recently, a PVC burden of 24 % was reported to be strongly associated with the presence of cardiomyopathy, although this cut-off value failed to identify every individual at risk of cardiomyopathy and the critical burden for some patients was noted to be lower.111

From the perspective of absolute PVC number, >20,000 PVCs in 24 hours was reported to correlate with a reduction in LVEF.117,118 Interestingly, LV dilation was associated with an increased burden of PVCs but not an absolute PVC frequency.118 Another study reported that dividing patients into groups based on total PVC burden from <1,000 PVCs/day, 1,000–10,000 PVCs/day and >10,000 PVCs/day yielded a prevalence of LV dysfunction of 4 %, 12 % and 34 %, respectively.119 It is clear that the likelihood of TIC development increases with increasing PVC burden. However, what is also clear is that PVC burden is not the only contributing factor to impaired LV function.
PVC QRS duration has been shown to be associated with the likelihood of TIC development. In one study, a PVC QRS duration of ≥140 milliseconds (msec) was an independent predictor of impaired LVEF.118 This finding was replicated at a second centre, which reported that PVC QRS duration was significantly greater in patients who developed PVC-induced cardiomyopathy as compared with those patients with PVCs but no LV dysfunction. In this study, a PVC QRS duration of >150 msec best differentiated patients with and without PVC-induced cardiomyopathy. An epicardial PVC origin was also independently associated with PVCinduced cardiomyopathy in these patients.120 Along similar lines, PVC QRS duration has been reported to independently predict reversibility of LV dysfunction, with a greater PVC duration predicting a lower likelihood of recovery. In this single-centre study, patients with a PVC QRS duration ≥170 msec were unlikely to normalise their LV function. There was also a gradient of PVC duration noted from those with normal, reversible, partially reversible and irreversible LV dysfunction.115
PVC coupling intervals have also been evaluated as a predictor of the development of TIC, although results have been inconsistent. One study reported that patients with PVC coupling intervals ≤600 msec had a lower mean LVEF than those patients with coupling intervals >600 msec.121 A more recent study reported that PVC interpolation predicted the development of PVC-induced cardiomyopathy independent of total PVC burden, albeit with an odds ratio only slightly above one.122
As with all forms of TIC, PVC-induced cardiomyopathy reverses with catheter ablation of PVCs in the majority of cases. In one series, LV function improved or normalised in more than 80 % of patients after a mean of three months following catheter ablation. Only a minority of these patients had echocardiograms performed within one week of ablation. However, in those patients where early echocardiography was performed, over 80 % demonstrated acute improvement in LV function.111 A more recent study reported that over half of all patients had a >25 % increase in LVEF at one-week following ablation and those patients with early improvement had a higher LVEF at 12 months of follow-up compared with patients without early improvement.123
It appears that complete elimination of PVCs is not necessary for improvement in LV function. One study reported an 80 % reduction of PVCs resulted in similar improvement in LV function compared with complete PVC elimination, although the magnitude of LVEF improvement did correlate with the decline in residual PVC burden.114 A more recent study confirmed these findings reporting that the majority of patients with idiopathic PVCs and LV dysfunction whose PVC burden was reduced by more than 80 % normalised their LVEF within four months.124 This finding is important given that total elimination of some idiopathic PVCs can be challenging due to origins difficult to ablate from a transvenous approach.
Imaging Studies in Tachycardia-induced Cardiomyopathy
Multiple animal models have suggested that the structural changes seen in TIC are unique from other forms of heart failure. Recent studies have looked at echocardiography and cardiac magnetic resonance (CMR) in humans with TIC in an attempt to better understand the underlying pathophysiology of TIC. It has been reported that patients with TIC and no other underlying structural disease have a smaller left ventricular end-diastolic diameter, LV volume when adjusted for body surface area (BSA) and LV mass index as compared with patients with idiopathic dilated cardiomyopathy at the time of initial presentation. In this study, LV end-diastolic dimension was the only independent predictor of TIC in multiple regression analysis and was felt to be the best echocardiographic predictor of TIC.125 Negative remodeling has been associated with TIC and has been shown to persist after resolution of the tachycardia. One study found that most echocardiographic abnormalities seen in TIC patients normalised following treatment of the causative tachycardia. However, when compared with gender, age and ejection fraction matched controls, patients with TIC had a significantly higher stroke volume, cardiac index, left ventricular end-systolic dimension, left ventricular end-systolic volume index and left ventricular end-diastolic index. The mean time interval between pre-treatment and post-treatment echocardiograms was 14 months in this group.126 These findings are consistent with previously discussed animal models of TIC, which found that after ejection fraction and other haemodynamic parameters normalised, LV volumes remained increased. However, in these animal models such persistent changes were only documented out to 12 weeks.33
Late gadolinium enhancement (LGE) on CMR has been shown to accurately identify areas of myocardial fibrosis and different patterns of LGE have been reported according to cardiomyopathy aetiology. One case series of patients with TIC related to ventricular arrhythmias found that LGE was present in only a single patient out of the 19 patients with TIC, imaged with CMR. Of note, five patients with primary cardiomyopathy were also scanned with CMR and four of the five patients had evidence of LGE.127 The investigators concluded that PVC-induced cardiomyopathy is less likely to have LGE on CMR as compared with other forms of cardiomyopathy. Consistent with this finding, a recent canine model of PVC-induced cardiomyopathy failed to demonstrate increased inflammation, fibrosis or changes in apoptosis or mitochondrial function.
Risk of Recurrence
It has been reported that recurrent tachycardia in patients with a prior history of TIC can lead to recurrent cardiomyopathy at a faster and more severe rate as compared with initial presentations, suggesting that although LVEF normalises, some structural abnormalities related to remodeling persist. In a cohort of 24 patients with TIC, five patients were noted to have recurrent tachycardia. In all five patients, an abrupt drop in ejection fraction was noted and all patients developed clinical heart failure within six months. Heart failure was reversed over a similar six month period once adequate rate control was established.128 A separate case series also reported recurrence in two of twelve consecutive patients during an average follow-up period of 53 months. In both patients the time from tachycardia symptom onset to the development of recurrent heart failure was less than two weeks, and in one patient was in a single day. Both patients had a decline in LVEF to a level similar to their initial presentation. After control of the arrhythmias, both patients again normalised their ejection fractions.129 An early study of TIC reported a severe deterioration in left ventricular systolic function in two of twelve patients after reversion to AF following cardioversion.130 The more rapid decline in LV function in patients with TIC reported above would suggest persistent structural abnormalities in such patients, which would also be supported by the echocardiographic findings described above. These findings also give additional weight to the importance of maintaining a strong medical regimen for TIC patients even after normalisation of their ejection fraction.
Risk of Sudden Death
There is little published data on the risk of sudden death in the setting of TIC. As noted above, animal models have reported conflicting results as to whether TIC leads to a proarrhythmic substrate increasing risk of ventricular arrhythmias. In the setting of clinical heart failure and depressed ejection fraction, one might suspect risks of malignant arrhythmias to be similar to other forms of heart failure. However, the more important question is whether an increased risk of sudden death in patients with a history of TIC persists even after normalisation of LV function. Case reports of patients with TIC who have died suddenly have been published in the literature. One centre reported three patients with a history of TIC who died suddenly. All three patients had TIC in the setting of AF and all patients’ LVEF normalised or near normalised with various rate control strategies including cardioversion, antiarrhythmic drugs (AADs) and an ablate and pace strategy. All patients died months to years after normalisation of their ejection fraction and per reports, had no heart failure symptoms nor symptoms of recurrent uncontrolled arrhythmia prior to their death. Of note, these three patients had significantly lower baseline LVEFs compared with the other patients with TIC.128 A separate site published a case report of a young man with incessant supraventricular tachycardia (SVT) who presented in congestive heart failure with evidence of a dilated cardiomyopathy on echocardiography. An endomyocardial biopsy revealed mild interstitial infiltration. His heart failure improved with medical therapy but his tachycardia persisted despite AADs. The patient died suddenly twenty days after discharge and it was postulated that his cardiomyopathy was tachycardia-induced. However, this report is limited by the fact that the patient did not have a clear diagnosis of TIC and could have had idiopathic dilated cardiomyopathy with concurrent SVT.131 A third study reported a patient with atrial flutter and cardiomyopathy, which recovered with heart rate control after eight months of therapy. The patients died suddenly four years after his initial presentation without preceding symptoms of tachycardia or CHF. At the time of this patient’s initial presentation with heart failure, he had the highest levels of BNP of all patients with TIC reported.129 Further prospective research is required to better determine whether there is a truly increased risk of ventricular arrhythmia and sudden death in patients with a history of resolved TIC.
Future Perspectives
Our current understanding of the mechanisms of LV dysfunction in the setting of TIC is limited. Animal models have helped us better evaluate the cellular and haemodynamic mechanisms underlying a pacinginduced model of TIC, but the applicability of these animal models to human disease has yet to be proven. The diagnosis of TIC remains challenging and a high index of suspicion is required given the potential for LV recovery with appropriate treatment. An aggressive approach to arrhythmia treatment, whether it be catheter ablation, AADs or rate control is important in patients with otherwise idiopathic cardiomyopathy when TIC is suspected. Further study of the risk factors for development of TIC will be an important area of future research to help better identify patients who are likely to develop cardiomyopathy in the setting of tachycardia. This may also help identify patients who are likely to have improvement in their cardiomyopathy with appropriate treatment of their tachycardia. A genetic approach to TIC risk is also an important area of future research given the wide spectrum of disease in patients with TIC. Recently, a paper reported an angiotensin-converting enzyme polymorphism that increased serum ace levels, which was more common in patients with TIC as compared with patients with tachycardia but no LV dysfunction.132 Imaging techniques may also be helpful at providing insight into the underlying mechanisms of TIC and may also help better characterise patients with cardiomyopathy of unknown aetiology and tachycardia at the time of presentation.